Factors affecting tumor recurrence after curative surgery for NSCLC: impacts of lymphovascular invasion on early tumor recurrence
Introduction
Non-small cell lung cancer (NSCLC) is one of the leading causes of cancer-related deaths worldwide (1). Though surgery is a potentially curative treatment for NSCLC up to stage IIIA disease, the risk of postoperative disease recurrence has been reported to be as high as 52% (2). Postoperative follow-up examinations are periodically performed to detect postoperative recurrence early, so that adequate treatment can be offered to improve survival rate. Because radiographic imaging is widely used for follow-up and there are numerous guidelines that have been published regarding the management of lung cancer (3), there should be an effort to avoid unnecessary medical treatment and reduce radiation exposure by adjusting follow-up plans. Although the TNM staging system for NSCLC is used to evaluate the extent of disease and guide management for patients (4), meticulous assessment of possible prognostic factors other than TNM stage is important for this reason. The purpose of this study was to identify factors that associated with recurrence and recurrence-free interval (RFI) after curative surgery for NSCLC.
Material and methods
Patient population
This study was approved by the institutional review board of our institution, and informed consent was waived due to the retrospective nature of the study. Between August 1999 and May 2013, a total of 218 patients underwent curative surgery for lung cancer at our institution, which is an urban tertiary care hospital.
Forty-six of 218 patients were excluded from the study for the following reasons: 26 underwent neoadjuvant therapy, 13 died or transferred to other hospital before the initial follow-up CT scan was obtained, image data or medical records were missing for five patients, one patient had surgically confirmed SCLC, one patient had history of both pancreatic and pulmonary adenocarcinoma with recurred adenocarcinoma of unspecified origin, and one 13-year-old patient was excluded by institutional review board because of absence of his parent’s permission.
The remaining 171 patients were included in this study. This patient population comprised 111 men and 60 women with a mean age of 63 years [median age: 64 years (range, 37-79 years)]. Median RFI was 20 months (range, 1-102 months). Other patient characteristics are summarized in Table 1.
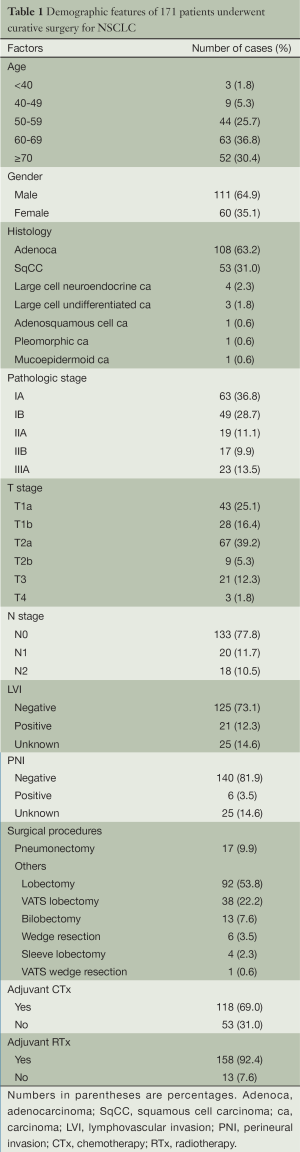
Full table
Pathologic analysis
Three pathologists interpreted lung tumor specimens during the study period. Hematoxylin and eosin stains were performed on all surgical specimens. Other pathologic studies, such as immunostains, were performed at the discretion of the pathologists. Lymphovascular invasion (LVI) was reported when tumor cells or emboli was identified in lymphatic or blood vessel lumens. Tumor involvement of epineurium was defined as perineural invasion (PNI) (5). Patients with unknown LVI or PNI status were grouped into the negative category (6). Postoperative pathologic reports from electronic medical records were reviewed. Data such as histologic type, pathologic TNM staging (pathologic staging), LVI, and PNI were collected. All postoperative pathologic staging for NSCLC at our institution has been performed according to the new TNM classification system of the American Joint Committee on Cancer (AJCC), 7th edition, since February 2010. Cases prior to this date were reevaluated according to the new TNM classification system by a radiologist with 4 years of experience of chest CT interpretation. Pathologic specimens of recurred lesions were obtained by bronchoscopic biopsy, core needle biopsy, and fine needle aspiration. Other lesions without pathologic confirmation were diagnosed by image analysis. RFI was defined as the time from the day of surgery to the date of the last normal follow-up for patients without recurrence, and to the date of first detection of a recurred lesion for patients with recurrence (7). For lesions that were not possible to distinguish between recurrence and second primary lung cancer, we considered the lesion to be recurred cancer, because it is often impractical to clarify disease recurrence from second primary lung cancer (7).
Image analysis
All CT scans were obtained using a 16-, 64- or 256-slice multidetector CT scanner (MX 8000 IDT, Phillips Medical System, Netherlands; Brilliance 64, Phillips Medical Systems, Israel; Somatom Definition Flash, Siemens Healthcare, Germany, respectively). All PET-CT scans were obtained using a large-bore, time-of-flight PET-CT scanner (Philips Gemini TF, Philips Healthcare, Andover, MA, USA). Routine follow-up chest CT for surveillance of recurrence was performed 3, 6, 12, 18, and 24 months after surgery, and then annually. Additional chest radiography, CT, or PET-CT scanning was performed depending on the clinical situation. CT and PET-CT scan data were collected from a picture archiving and communication system (PACS; Infinitt Healthcare Co. Ltd., Seoul, Korea). Consensus review of preoperative and follow-up CT and PET-CT images were performed by two radiologists, one with 16 years and the other with 4 years of experience in chest radiology.
In patients without pathologic confirmation, recurrence was presumed when there was any newly identified or enlarging soft tissue lesion or lymph node enlargement with more than one of the following: intense FDG uptake on PET-CT scans taken within a month of detection, either persistence or aggravation on the next follow-up study. Lymph node enlargement was defined when the short axis diameter was greater than 1 cm on CT scans.
In this study, recurrence was classified as locoregional or distant recurrence. Locoregional recurrence was defined when disease recurred at the surgical resection margin or regional lymph nodes (ipsilateral hilar and/or mediastinum) (3,6). The location of locoregional recurrence was subclassified into stump, endobronchial, regional lymph node, and pleural invasion directly from recurrent mass. Pleural seeding and recurrence in the remaining ipsilateral or contralateral lung were regarded as distant metastasis (8). The location of distant recurrence was subclassified into ipsilateral and contralateral lung, distant lymph node, ipsilateral pleura incontinuous to surgical margin, contralateral pleura, and distant organ. When locoregional and distant recurrences were concurrently identified (concurrent cases), the two were counted separately.
Statistical analysis
Several clinicopathologic parameters were evaluated in this study: histologic type, pathologic stage, T stage, N stage, LVI, PNI, surgical procedure, adjuvant chemotherapy, and adjuvant radiotherapy. Surgical procedure, adjuvant chemotherapy and adjuvant radiotherapy were dichotomous: pneumonectomy or others, received or not. Because the clinicopathologic parameters did not show a normal distribution, the Mann-Whitney U test and Kruskal-Wallis test were applied to each clinicopathologic factor to investigate the significance of differences in mean RFI according to the factor.
Univariate and multivariate analyses were performed to examine factors affecting recurrence using the Kaplan-Meier method and Cox proportional hazards model, respectively. Log-rank test was used for univariate analysis of recurrence differences. Clinicopathologic parameters were included in univariate analysis. Gender, age, histologic type, and factors with P≤0.1 on univariate analysis (pathologic stage, T stage, N stage, LVI, and adjuvant chemotherapy) were included in multivariate analysis of overall, locoregional, and distant recurrences. Fisher’s exact test was performed to identify preferred initial recurrence location on recurrent cases with LVI, compared with recurrent cases without LVI.
We also performed Kaplan-Meier analysis of the relationship between LVI and distant recurrence with stratification according to pathologic stage to identify whether pathologic stage was a confounding factor. Association between LVI and pathologic stage was evaluated by linear association.
All statistical analyses were performed using SPSS version 19.0 (IBM-SPSS, Chicago, IL, USA). All differences with P<0.05 on two-tailed tests were considered to be statistically significant.
Results
Among 171 patients, 53 (31.0%) had recurrent lesions, which were diagnosed by pathologic confirmation in 11 patients and imaging data in the remaining 42 patients. There were no significant differences in mean RFI according to clinicopathologic factors except for LVI (P<0.001), which was associated with three-fold increase in mean RFI (31.43 vs. 10.29 months). Surgical procedure showed borderline significance (P=0.057). Differences in mean RFI according to clinicopathologic factors are summarized in Table 2.
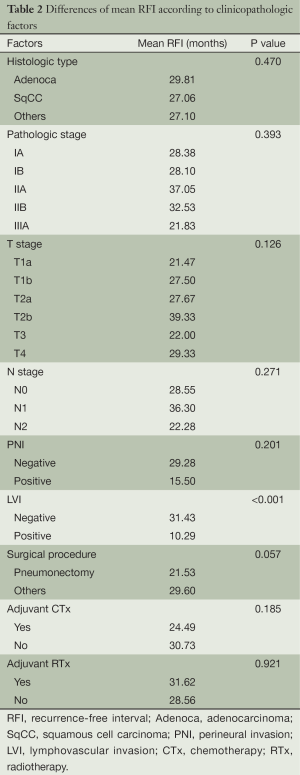
Full table
Univariate analysis revealed the following factors that were significantly associated with recurrence rate: pathologic stage (P=0.003), T stage (P=0.012), N stage (P=0.016), LVI (P<0.001), pneumonectomy (P<0.001), and adjuvant chemotherapy (P=0.011) (Figure 1). Histologic type and PNI did not show statistical significance (P=0.514 and 0.701, respectively). Patients with LVI had a lower 2-year recurrence-free rate than patients without LVI by one-third (44.6% vs. 14.9%, Figure 2). There was an approximately 2-fold difference in 2-year recurrence-free rate between the highest and lowest tiers of other significant factors on univariate studies (pathologic stage IA vs. IIIA, 0.874 vs. 0.381; T stage T1a vs. T4, 0.920 vs. 0.500; N stage N0 vs. N2, 0.780 vs. 0.403; pneumonectomy vs. others, 0.306 vs. 0.772; received adjuvant chemotherapy vs. not, 0.599 vs. 0.784).
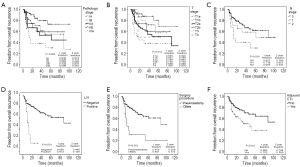
Multivariate analysis of overall recurrence revealed that T stage (P=0.045), N stage (P=0.044), and LVI [P<0.001, hazards ratio (HR): 4.76, 95% confidence interval: 2.08-10.90] were independent predictors of overall recurrence. Pathologic stage (P=0.068) showed borderline significance. Gender, age, histologic type, surgical procedure, adjuvant chemotherapy were not statistically significant (P=0.562, 0.603, 0.739, 0.124, and 0.748 respectively). Among these, there was a substantial decrease in the statistical support for an association between surgical procedure and RFI compared to the univariate analysis. On multivariate analysis of locoregional recurrence, only pathologic stage (P=0.005) was significant. Histologic type, and surgical procedure (P=0.081 and 0.079, respectively) showed borderline significance. Gender, age, T stage, N stage, LVI, and adjuvant chemotherapy did not show statistical significance (P=0.386, 0.110, 0.351, 0.549, and 0.813, respectively). LVI and T stage were demonstrated to be the significant factors affecting distant recurrence (P<0.001, P=0.040, respectively). Gender, age, histologic type, N stage, surgical procedure, and adjuvant chemotherapy did not show statistical significance (P=0.192, 0.877, 0.273, 0.686, 0.375, and 0.748, respectively). Pathologic stage could not be included in this analysis because it did not converge. Results of multivariate analyses are summarized in Table 3.
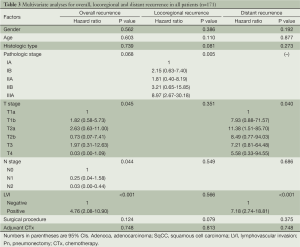
Full table
On the analysis with Fisher’s exact test, there was no preferred initial recurrence location that showed statistical significance on LVI positive cases.
Discussion
There are some reports that radiation therapy in patients with early stage recurrence can increase survival rate (2,9,10). Considering that the postoperative recurrence rate for NSCLC has been reported to be as high as 52% (2), early detection of recurrence is critical to allow effective local treatment. Pathologic staging is known as the most powerful prognostic factor for lung cancer (11). According to the retrospective study of Sawada et al., the combination of T stage, N stage, and LVI was correlated with the risk of recurrence (4). Another study identified pneumonectomy as a significant variable, although the authors attributed this to technical advancement (12). In the current study, we evaluated these parameters as independent factors that could be associated with NSCLC recurrence.
T stage, N stage, and LVI had a significant impact on the overall recurrence rate in multivariate analysis, consistent with previous studies (4,11,12). Surgical procedure was a significant factor in univariate analysis of overall recurrence and showed borderline significance on the Mann-Whitney U test. That was probably due to confounding effects of other clinicopathologic factors, as there was a substantial decrease in significance in multivariate analysis of overall recurrence. However, further evaluation is warranted considering the results of a previous study (12).
One of the reasons why the risk of local recurrence is poorly defined is that the definition of locoregional recurrence varies widely among studies. Martini and colleagues defined local recurrence as evidence of tumor within the same lung or at the bronchial stump, and regional recurrence as clinically or radiologically manifest disease in the mediastinum or in the supraclavicular lymph nodes (13). Kelsey et al. (7) and Higgins et al. (6) defined it as disease recurrence at the surgical resection margin, ipsilateral hilum, or mediastinum. This definition was adopted for practical reasons as it includes typical radiation fields in a postoperative setting (7). Although the temporal relation between locoregional and distant recurrence is uncertain in concurrent cases, it can be assumed that locoregional recurrence occurred first, and might be the cause of the distant recurrence (7). Therefore, we counted locoregional recurrence and concurrent distant recurrence separately, despite the fact that local treatment such as surgery or radiation therapy cannot be used in such cases. In the study of Jang and colleagues (8), the locoregional pattern differed significantly according to histologic type. Its borderline significance in multivariate analysis of locoregional recurrence in this study (P=0.081) may be consistent with the results of the previous study.
Vessel invasion has already been demonstrated to be a prognostic pathological marker for survival in breast, colorectal, and head and neck cancers (14). Although LVI is not included in the TNM staging system currently used, several recent studies reported LVI as an adverse prognostic factor for NSCLC recurrence (4-6,14,15). Higgins et al. reported LVI as an adverse prognostic factor for the development of distant recurrence in their study of the patients with NSCLC stage IA to IIIB (6). In the current study, we demonstrated that LVI had a significant association with high distant recurrence rate (P<0.001) in contrast to other pathological factors. This result is consistent with the previous study of Higgins and colleagues (6).
In this study, the result of distant recurrence include T stage as a significant independent factor, which is in agreement with the study of Hung et al. (16), which concludes that T stage increases the risk of distant metastasis on stage I NSCLC. However, further study with NSCLC of advanced stages is warranted as this study targets all operable stages.
The retrospective study of Al-Alao and colleagues of 457 patients with stage I and II NSCLC identified lymphatic vessel invasion, excluding intratumoral vascular invasion, as a significant predictor of early tumor recurrence (14). Univariate analysis revealed a significant difference in 5-year disease-free survival according to the presence or absence of LVI. Shiono et al. studied 547 patients with stage II to IV NSCLC and reported LVI as an independent significant factor for earlier recurrence by demonstrating a significant difference in postoperative recurrence survival on univariate analysis (17). We included all operable stages of NSCLC in our study and defined LVI as tumor cells or emboli in at least one lymphatic or blood vessel. We found a 3-fold difference between the 2-year recurrence-free survival of the LVI positive and negative groups, which was statistically significant (44.6% vs. 14.9%, P<0.001). This finding is consistent with that of previous studies (14,17). Considering this result and identification of LVI as a significant prognostic factor for overall recurrence in multivariate analysis, the significantly shorter mean RFI of the LVI positive group than that of the LVI negative group on the Mann-Whitney U test was expected. The lack of significance of other factors that were significant in multivariate analysis of overall recurrence (pathologic stage, T stage, and N stage) indicates that LVI may be one of the factors explaining the high incidence of recurrence within 2 years in NSCLC patients treated by curative surgery (3,18). Therefore, detection of LVI may form the basis for adjuvant therapy (14) and meticulous radiologic follow-up during the early postoperative period in patients with any operable stage of primary NSCLC.
In our study, there was no significantly preferred initial recurrence location on LVI positive cases. This is probably because lymphatics and blood vessels might be reservoir of viable tumor cells and common route for tumor spread.
The limitations of this study are as follows. As immunohistochemical stainings were not performed in all cases, the underestimation of LVI and PNI was inevitable. However, the definitions of LVI and PNI were identical to several previous studies (5,7,14,15). Because this retrospective study was carried out on a relatively small group of patients within a single institution, statistical power to make universal conclusions is lacking (14). Pathologic stage could not be included in multivariate analysis of distant recurrence. That was due to the small number of patients although sufficient patients were analyzed to be able to demonstrate a relationship between LVI and high distant recurrence rate. However, pathologic stage did not appear to be a confounding factor; Kaplan-Meier analysis of the association between LVI and distant recurrence stratified according to pathologic stage was highly significant (P<0.001) and was not significantly associated with distant recurrence (P=0.215). Further studies with larger numbers of patients are warranted. Additionally, more than half of the cases were stage I cases, indicating that our findings may not be widely generalized. Absence of survival analysis was also a limitation of this study, although it is known that the prognosis of patients with NSCLC recurrence after surgery is poor (19).
In conclusion, T and N stages were significantly related to overall recurrence, and pathologic TNM staging showed borderline significance for overall recurrence, consistent with previous studies (4,11,12). LVI was associated with both high overall and distant recurrence rates, as well as early tumor recurrence after curative surgery for NSCLC. Meticulous radiologic follow-up might be helpful for LVI positive cases.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yamazaki K, Sugio K, Yamanaka T, et al. Prognostic factors in non-small cell lung cancer patients with postoperative recurrence following third-generation chemotherapy. Anticancer Res 2010;30:1311-5. [PubMed]
- Zimmermann FB, Molls M, Jeremic B. Treatment of recurrent disease in lung cancer. Semin Surg Oncol 2003;21:122-7. [PubMed]
- Colice GL, Rubins J, Unger M, et al. Follow-up and surveillance of the lung cancer patient following curative-intent therapy. Chest 2003;123:272S-83S. [PubMed]
- Sawada S, Yamashita N, Suehisa H, et al. Risk factors for recurrence after lung cancer resection as estimated using the survival tree method. Chest 2013;144:1238-44. [PubMed]
- Yilmaz A, Duyar SS, Cakir E, et al. Clinical impact of visceral pleural, lymphovascular and perineural invasion in completely resected non-small cell lung cancer. Eur J Cardiothorac Surg 2011;40:664-70. [PubMed]
- Higgins KA, Chino JP, Ready N, et al. Lymphovascular invasion in non-small-cell lung cancer: implications for staging and adjuvant therapy. J Thorac Oncol 2012;7:1141-7. [PubMed]
- Kelsey CR, Marks LB, Hollis D, et al. Local recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patients. Cancer 2009;115:5218-27. [PubMed]
- Jang KM, Lee KS, Shim YM, et al. The rates and CT patterns of locoregional recurrence after resection surgery of lung cancer: correlation with histopathology and tumor staging. J Thorac Imaging 2003;18:225-30. [PubMed]
- Jeremic B, Shibamoto Y, Milicic B, et al. External beam radiation therapy alone for loco-regional recurrence of non-small-cell lung cancer after complete resection. Lung Cancer 1999;23:135-42. [PubMed]
- Kagami Y, Nishio M, Narimatsu N, et al. Radiotherapy for locoregional recurrent tumors after resection of non-small cell lung cancer. Lung Cancer 1998;20:31-5. [PubMed]
- Hashizume S, Nagayasu T, Hayashi T, et al. Accuracy and prognostic impact of a vessel invasion grading system for stage IA non-small cell lung cancer. Lung Cancer 2009;65:363-70. [PubMed]
- Ichinose Y, Kato H, Koike T, et al. Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: questionnaire survey of the Japan Clinical Oncology Group to plan for clinical trials. Lung Cancer 2001;34:29-36. [PubMed]
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [PubMed]
- Al-Alao BS, Gately K, Nicholson S, et al. Prognostic impact of vascular and lymphovascular invasion in early lung cancer. Asian Cardiovasc Thorac Ann 2014;22:55-64. [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. Prognostic impact and initial recurrence site of lymphovascular and visceral pleural invasion in surgically resected stage I non-small-cell lung carcinoma. Eur J Cardiothorac Surg 2013;44:e200-6. [PubMed]
- Hung JJ, Jeng WJ, Hsu WH, et al. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol 2012;7:1115-23. [PubMed]
- Shiono S, Kanauchi N, Yanagawa N, et al. Stage II-IV lung cancer cases with lymphovascular invasion relapse within 2 years after surgery. Gen Thorac Cardiovasc Surg 2014;62:112-8. [PubMed]
- Bogot NR, Quint LE. Imaging of recurrent lung cancer. Cancer Imaging 2004;4:61-7. [PubMed]
- Saisho S, Yasuda K, Maeda A, et al. Post-recurrence survival of patients with non-small-cell lung cancer after curative resection with or without induction/adjuvant chemotherapy. Interact Cardiovasc Thorac Surg 2013;16:166-72. [PubMed]


