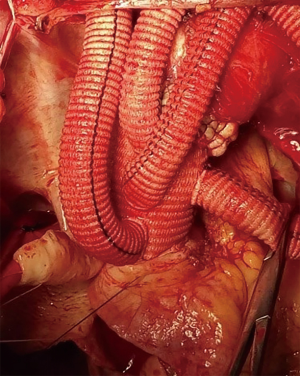
“Aorta-clamp” technique for surgical repair of acute type A aortic dissection—5 min circulatory arrest at 30 °C
Introduction
Acute type A aortic dissection (AAAD), the most challenging catastrophic disease in cardiothoracic surgery, is a highly fatal disease associated with high morbidity and mortality (1), and emergent surgical treatment is recommended for all patients to repair the proximal aorta, and prevent rupture and consequent cardiac tamponade (2). With advances in anesthesia and cardiopulmonary bypass (CPB), many surgical approaches have been developed including Sun’s procedure (3), the arch open technique (4), the island technique (5), as well as the single-branched stent graft (6), the Y-graft (7) and the trifurcated branched grafts (8). One fundamental element, deep hypothermic circulatory arrest (HCA), is known to be a major cause of many intraoperative and postoperative complications (9, 10), but is used in all of these surgical procedures. HCA is also associated with a greater risk of postoperative low cardiac output syndrome (11). Therefore, the development of a novel surgical procedure that can extensively replace the dissected aorta and avoid the negative impacts of a long surgical period and deep HCA remains a high priority. In the present study, we report our novel surgical strategy, an “aorta-clamp” technique with continuous bilateral antegrade cerebral perfusion, which significantly reduces the time of circulatory arrest to 5 min and raises the temperature of HCA to mild hypothermia (30 °C) during circulatory arrest. This study aims to evaluate the safety and efficiency of this technique for the surgical repair of AAAD.
Methods
Patient recruitment
From November 2014 to August 2016, 59 consecutive patients including 47 men and 12 women (average age 51.3±10.9 years, ranging from 28 to 70 years) with AAAD were enrolled into this study. All diagnoses were made by preoperative computed tomography (CT) and echocardiography examinations. Seven patients had Marfan syndrome and 30 patients had a history of hypertension. Patients with only ascending aorta involvement or a primary entry tear which can be repaired by hemi-arch replacement were excluded. All patients underwent total aortic arch replacement combined with implantation of an elephant trunk stent graft using the “aorta-clamp” technique under CPB with continuous bilateral antegrade cerebral perfusion. All patients received surgery within 2 weeks after onset of chest pain. This study and the technique used were approved by the Ethics Committee of Guangdong People Hospital (No. A201338) and written informed consent was obtained from each patient before surgery.
Surgical technique
After induction of general anesthesia, the patient was placed in the supine position. The arterial blood pressure of both the left upper and lower limbs was monitored and a probe for transesophageal echocardiography was inserted.
The aortic arch and heart were exposed via a median sternotomy. The femoral artery, right axillary artery, innominate artery, left common carotid artery and left subclavian artery were dissociated. CPB was established by a two-stage cannula via the right atrium and an arterial cannula placed in the femoral artery. CPB flow was maintained between 2.2 and 2.4 L·m−2·min−1. Systemic cooling to a bladder temperature of 30 °C was commenced.
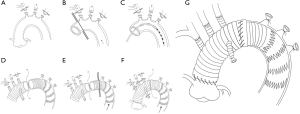
The innominate artery, the left common carotid artery, and the left subclavian artery were ligated and transected after bilateral antegrade cerebral perfusion was established through two arterial cannulas placed in the right axillary artery and the left common carotid artery. The aortic arch was divided as fully as possible (Figure 1A). The ascending aorta was clamped just proximal to the innominate artery and transected 1.5 to 2 cm proximal to the clamp (Figure 1B). Myocardial protection was achieved by intermittent antegrade perfusion of cold blood cardioplegic solution. Proximal manipulations, such as aortic valve repair, sinus of Valsalva reconstruction, coronary artery bypass grafting, the Bentall, Cabrol or David procedure were performed accordingly. The patients were then placed in a head-down position and CPB via the femoral artery for the lower body was discontinued. The brain was continually perfused at a flow rate of 8 to 10 mL·kg−1·min−1 through the right axillary and left common carotid artery. The aortic cross clamp was removed, and the distal ascending aorta and proximal aortic arch were fully divided. A compressed stent graft (MicroPort Medical Co., Ltd. Shanghai, China, diameter: 23–28 mm, length: 120 or 150 mm) was inserted into the true lumen of the aortic arch and descending aorta and deployed (Figure 1C). The posterior wall of the transected distal ascending aorta containing the intraluminal stent graft was anastomosed to a 4-branched graft by 4 to 5 stitches using 4-0 polypropylene (Figure 1D). The distal ascending aorta containing the intraluminal stent graft was then clamped, known as the “aorta clamp” technique, and perfusion of the lower body was immediately resumed through the femoral artery (Figure 1E). Anastomosis of the distal ascending aorta containing the intraluminal stent graft to the 4-branched graft was then completed. After finishing the proximal aortic anastomosis, the clamp was removed in order to reduce cardiac ischemia time (Figure 1F). Systemic re-warming was started as soon as the left common carotid artery anastomosis was completed. The left subclavian artery and innominate artery anastomoses were then performed in order (Figure 1G and Figure 2). Hemostasis was carried out in a standard fashion.
AAAD patients without branch-vessel abnormalities or surgical contradictions were included. The selection criteria for employing this technique included: (I) a primary entry tear which could not be repaired by hemi-arch replacement, (II) complications related to arch or arch vessel involvement (such as malperfusion, rupture or aneurysm >55 mm) and (III) patients with connective tissue disorders such as Marfan syndrome.
Neurological and renal function monitoring
The neurological and renal functions were closely monitored before and after surgery. To determine whether this procedure could reduce end-organ damage, the levels of serum creatinine (sCr), a simple and sensitive indicator of acute kidney injury (AKI), were detected before and immediately after surgery as well as 24 and 48 h later. An elevation of sCr of 26.5 µmol/L or a 50% increase above normal sCr level (≤115 µmol/L) was considered preoperative or postoperative AKI (12). Unresolved postoperative AKI before discharge in patients without preoperative AKI was the end point of this study. Neuropsychological evaluation was performed if neuropsychological symptoms, including delirium or confusion, occurred and further examination such as CT scanning was carried out if required.
Statistical analysis
Results are expressed as mean ± standard deviation. Percentages in parentheses were calculated based on total patient numbers. Statistical analysis was performed using the Student t-test with IBM SPSS 24.0 software (IBM Corp., USA). A P value less than 0.05 was considered significant.
Results
Twenty-three patients had preoperative AKI as evidenced by increased levels of sCr (Table 1). All patients underwent surgery within 2 weeks after onset of chest pain, and 50 patients underwent surgery within 48 h.
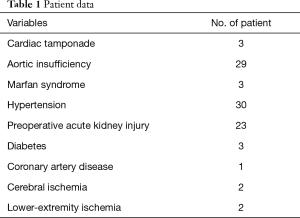
Full table
The average operating time was 7.3±1.7 h. The mean CPB time was 217.4±37.5 min, mean aortic cross-clamp (ACC) time was 147.4±22.5 min, and mean rewarming time was 46.8±6.9 min. The mean HCA time was 4.9±1.0 min (range, 4 to 8 min). Forty-nine patients (83.3%) underwent surgery with an HCA time less than 5 min. All patients received a blood transfusion (average 486±23 mL). Average drainage from the mediastinal chest tube was 458±140 mL in the first 24 h. Concomitant procedures are summarized in Table 2.
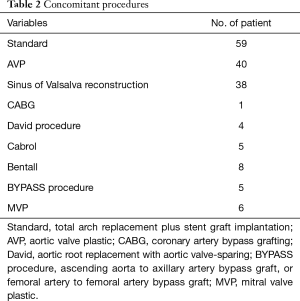
Full table
Studies have shown that mortality was increased in patients with AKI during thoracic surgery (12). By comparing patients with and without preoperative AKI, we found that operating time, CPB, ACC, and rewarming time were significantly prolonged in the AKI group. Units of blood transfused and drainage from the mediastinal chest tube were also greater in the AKI group. There was no difference in age and HCA between patients with and without AKI (Table 3).
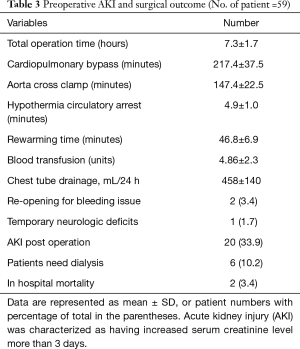
Full table
Of the 23 patients with preoperative AKI, 11 patients had mild kidney dysfunction (sCr <172.5 µmol/L, 1.5-fold of the normal value) and in five patients, kidney function returned to normal the first day after surgery. Twelve patients had severe kidney injury characterized by an increase in sCr of more than 1.5-fold (>172.5 µmol/L) (13). Six (10.2%) of these patients required postoperative dialysis and two patients died of multiple organ failure induced by postoperative infection. The postoperative mortality rate was 3.4%. The other four patients who needed dialysis fully recovered 3 to 11 days after operation. The sCr level returned to normal in 15 patients with preoperative AKI before discharge. Two patients showed increased sCr levels (119 and 121 µmol/L, respectively) on the first day after surgery, which returned to normal on the second day. No difficulties in achieving hemostasis were encountered in these patients. Two patients (3.4%) required re-exploration to correct postoperative bleeding due to fresh blood drainage from the mediastinal chest tube of more than 200 mL in the first hour. One of these patients received dialysis but died due to multiple organ failure (Table 4). Of the 59 patients, only one (1.7%) exhibited temporary neurologic deficits.
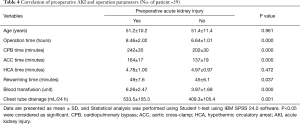
Full table
All patients who survived to hospital discharge were followed up. The average follow-up period was 11.9 months (range, 6 to 25 months). No severe complications related to the surgery or AAAD itself were reported. There were no late deaths and no reoperations were required. All patients resumed normal activities.
Postoperative CT scanning was conducted to determine the patency of the anastomotic sites in all patients. The false lumen around the stent graft was obliterated by thrombi in 51 patients (89.5%), partially by thrombi in five patients (8.8%), and remained patent in one patient (1.7%).
Discussion
Deep HCA greatly influences the surgical outcome of AAAD via the following mechanisms. Firstly, deep HCA affects the hemostatic system (12) that is already compromised due to the disease (14) and surgery (12). Hemostasis is critical in arch surgery and is directly related to intraoperative and postoperative mortality. Secondly, deep HCA affects end-organ function such as the kidney (15, 16), which leads to a high rate of postoperative dialysis and thus affects long-term survival (17). Third, a long period of deep HCA causes neural function damage (18), which has been proved to be an independent risk factor for postoperative death and increased medical expenses (19). Therefore, the development of a novel surgical technique that can avoid the complications of deep HCA during AAAD repair may improve the outcome of these patients. Here we report our new surgical procedure, known as the “aorta-clamp” technique, which has the following advantages in AAAD repair: (I) lower body ischemia time is significantly reduced to 5 min and the temperature of HCA is raised to 30 °C, which greatly preserves the hemostatic system and neural function, and avoids postoperative complications such as end-organ damage; (II) due to mild HCA, the recommended long stepwise rewarming process (20) can be shortened; and (III) hemostasis and bleeding control during surgery can be achieved with less time and effort.
We have demonstrated that the spinal cord can withstand hypoxia up to 10 min at 36 °C without any functional impact (21). A study carried out in pigs also showed that spinal cord damage only occurs after 90 min of selective cerebral perfusion at moderate hypothermia (22), suggesting that the spinal cord can tolerate relatively high temperature HCA. Moderate hypothermia, which is employed clinically in circulatory arrest, attenuates the risk of neurological complications, but increases the incidence of renal dysfunction and failure (23). To solve this problem, we raised the temperature of HCA to 30 °C and shortened the circulatory arrest time to around 5 min by clamping the proximal site of the compressed stent graft, known as the “aorta-clamp” technique. Using this technique, we dramatically shortened the ischemia time of the lower body under a mild temperature. The key points in this procedure are as follows: (I) the ACC is placed close to the innominate artery and the aorta is fully transected 1.5 to 2 cm proximal to the clamp to ensure an adequate length for subsequent surgery; (II) the posterior wall of the transected aorta is anastomosed to the 4-branched graft before it is clamped, which keeps the stent graft in position and allows easy clamping; (III) circulation of the lower body is immediately resumed after clamping the transected aorta, which decreases the ischemia time of lower body organs including the thoracic and lumbar spinal cord; (IV) continuous bilateral anterograde cerebral perfusion is set at 8 to 10 mL/kg−1.min−1, which provides adequate perfusion of the brain and cervical spinal cord to cope with potential injury due to high temperature HCA (30 °C); (V) the stent graft must be used to provide a support structure for the fragile aorta caused by aortic dissection. The stent graft used in this technique needs to be longer than usual (120 to 150 mm) to cover intimal tears in the descending aorta and have adequate length at the proximal end for clamping and anastomosis. In the case of a large false lumen, it would be difficult to stitch the stent graft to the transected aorta and bleeding from the suture site might be encountered. To this end, continuous suture to reinforce the suture line is recommended.
Using this technique, excellent surgical outcomes were achieved. The mean ACC time and CBP time were 164 and 241 min, respectively, which are similar (or shorter than) to other reported techniques. There were only two in-hospital deaths (3.4%) due to postoperative infection. This is lower than that in other reports, such as Kazui et al. who reported an in-hospital mortality rate of 17% (24), the German Registry for Acute Aortic Dissection Type A showed a rate of 25.7% (25), and the International Registry of Acute Aortic Dissection showed a rate of 23.7% (26) following total arch replacement. Additional studies are needed to compare our outcomes as our patients were relatively younger (average age 51.2 years, ranging from 28 to 70 years), which may have been due to high blood pressure in some patients, although Trimarchi et al. indicated that only age 70 years or more is an independent predictor of mortality (26). Six patients (10.2%) in this series required dialysis, and all had AKI before surgery and thus we do not think the requirement for dialysis was related to surgery. Only two patients without preoperative AKI had mildly increased sCr on the first day after surgery and their kidney function returned to normal on the second day. Although age is not related to AKI, and preoperative AKI does not affect HCA time, our data suggest that preoperative AKI negatively influences other factors (Table 4). Therefore, preoperative AKI may be an indicator of surgical difficulty and poor prognosis. One patient exhibited neurological complications (1.7%), which is lower than that in other reports (27, 28). Follow-up CT scans showed good anastomotic patency (Figure 3). Thrombosis and remodeling of the false lumen in the distal aorta were similar to those in other’s report (29).
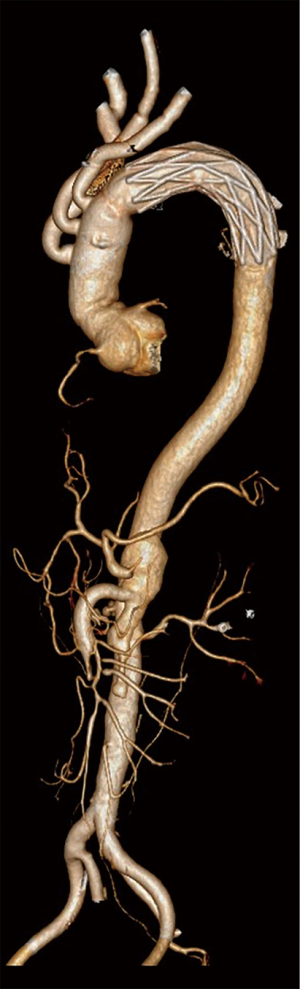
To date, surgical treatment of AAAD is still very challenging. There is no conclusive evidence of which surgical method is superior. There are also reports demonstrating excellent outcomes following surgical treatment of AAAD (30). In Park’s study (31), favorable outcomes were achieved by employing customized surgical approaches combined with several adjunctive procedures. Surgeons’ experience and institutional patterns still play important roles in treatment. Therefore, further studies are required. In this, we provide an alternative surgical approach, which may bring some insight into this topic, especially regarding the HCA.
Conclusions
The “aorta-clamp” technique is a safe and feasible strategy for the surgical repair of AAAD and provides a new perspective for colleagues who are facing similar challenges. Using this technique, the circulatory arrest time is reduced to 5 min and the temperature is raised to 30 °C, which preserved neurological and renal function, reduced systemic rewarming time and intraoperative hemostasis, and thus resulted in a reduction in operating time. Consequently, favorable overall outcomes with satisfactory safety as well as reasonable efficiency were achieved.
Acknowledgments
Funding: Natural Science Foundation of Guangdong Province China (grant number 2016A030313792), Medical Science Research Foundation of Guangdong Province China (grant number 2016115114137325), and Chinese Medicine Research Foundation of Guangdong Province China (grant number 20161003).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study and the technique used were approved by the Ethics Committee of Guangdong People Hospital (No. A201338) and written informed consent was obtained from each patient before surgery.
References
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Kremer J, Preisner F, Dib B, et al. Aortic arch replacement with frozen elephant trunk technique - a single-center study. J Cardiothorac Surg 2019;14:147. [Crossref] [PubMed]
- Apaydin AZ, Buket S, Posacioglu H, et al. Perioperative risk factors for mortality in patients with acute type A aortic dissection. Ann Thorac Surg 2002;74:2034-9. [Crossref] [PubMed]
- Chen LW, Wu XJ, Dai XF, et al. Total arch repair for acute type A aortic dissection with open placement of a modified triple-branched stent graft and the arch open technique. J Cardiothorac Surg 2014;9:135. [Crossref] [PubMed]
- Shrestha M, Martens A, Behrendt S, et al. Is the branched graft technique better than the en bloc technique for total aortic arch replacement? Eur J Cardiothorac Surg 2014;45:181. [Crossref] [PubMed]
- Chen LW, Wu XJ, Lu L, et al. Total arch repair for acute type A aortic dissection with 2 modified techniques: open single-branched stent graft placement and reinforcement of the dissected arch vessel stump with stent graft. Circulation 2011;123:2536-41. [Crossref] [PubMed]
- LeMaire SA, Price MD, Parenti JL, et al. Early outcomes after aortic arch replacement by using the Y-graft technique. Ann Thorac Surg 2011;91:700-7; discussion 707-8. [Crossref] [PubMed]
- Tang GH, Masashi K, Ramin M, et al. Trifurcated graft replacement of the aortic arch: state of the art. J Thorac Cardiovasc Surg 2015;149:S55-8. [Crossref] [PubMed]
- Livesay JJ, Cooley DA, Reul GJ, et al. Resection of Aortic Arch Aneurysms: A Comparison of Hypothermic Techniques in 60 Patients. Ann Thorac Surg 1983;36:19-28. [Crossref] [PubMed]
- Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: A propensity score analysis. J Thorac Cardiovasc Surg 2007;133:501-9. [Crossref] [PubMed]
- Yoo JS, Kim JB, Joo Y, et al. Deep hypothermic circulatory arrest versus non-deep hypothermic beating heart strategy in descending thoracic or thoracoabdominal aortic surgery. Eur J Cardiothorac Surg 2014;46:678-84. [Crossref] [PubMed]
- Ye M, Dai Q, Zheng J, et al. The Significance of Post-operative Creatinine in Predicting Prognosis in Cardiac Surgery Patients. Cell Biochem Biophys 2014;70:587-91. [Crossref] [PubMed]
- Kato A, Ito E, Kamegai N, et al. Risk factors for acute kidney injury after initial acute aortic dissection and their effect on long-term mortality. Ren Replace Ther 2016;2:53. [Crossref]
- Coselli JS, Lemaire SA. Aortic arch surgery: principles, strategies, and outcomes. Wiley-Blackwell, 2008:151-2.
- Guan XL, Wang XL, Liu YY, et al. Changes in the Hemostatic System of Patients With Acute Aortic Dissection Undergoing Aortic Arch Surgery. Ann Thorac Surg 2016;101:945. [Crossref] [PubMed]
- Song SW, Yoo KJ, Shin YR, et al. Effects of intermittent lower body perfusion on end-organ function during repair of acute DeBakey type I aortic dissection under moderate hypothermic circulatory arrest. Eur J Cardiothorac Surg 2013;44:1070-4; discussion 1074-5. [Crossref] [PubMed]
- Mori Y, Sato N, Kobayashi Y, et al. Low levels of urinary liver-type fatty acid-binding protein may indicate a lack of kidney protection during aortic arch surgery requiring hypothermic circulatory arrest. J Clin Anesth 2014;26:118-24. [Crossref] [PubMed]
- Swaminathan M, Hudson CC, Phillips-Bute BG, et al. Impact of early renal recovery on survival after cardiac surgery-associated acute kidney injury. Ann Thorac Surg 2010;89:1098-104. [Crossref] [PubMed]
- Reich DL, Uysal S, Sliwinski M, et al. Neuropsychologic outcome after deep hypothermic circulatory arrest in adults. J Thorac Cardiovasc Surg 1999;117:156. [Crossref] [PubMed]
- Okita Y, Minatoya K, Tagusari O, et al. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg 2001;72:72-9. [Crossref] [PubMed]
- Alam HB, Rhee P, Honma K, et al. Does the rate of rewarming from profound hypothermic arrest influence the outcome in a swine model of lethal hemorrhage? J Trauma 2006;60:134-46. [Crossref] [PubMed]
- Fan X, Asai T, Morioka K, et al. Measurement of glucose metabolism in rat spinal cord slices with dynamic positron autoradiography. Nucl Med Biol 2009;36:183-9. [Crossref] [PubMed]
- Etz CD, Luehr M, Kari FA, et al. Selective cerebral perfusion at 28 degrees C--is the spinal cord safe? Eur J Cardiothorac Surg 2009;36:946-55. [Crossref] [PubMed]
- Kazui T, Washiyama N, Bashar AH, et al. Surgical outcome of acute type A aortic dissection: analysis of risk factors. Ann Thorac Surg 2002;74:75-81. [Crossref] [PubMed]
- Kornilov IA, Sinelnikov YS, Soinov IA, et al. Outcomes after aortic arch reconstruction for infants: deep hypothermic circulatory arrest versus moderate hypothermia with selective antegrade cerebral perfusion. Eur J Cardiothorac Surg 2015;48:e45-50. [Crossref] [PubMed]
- Trimarchi S, Eagle KA, Nienaber CA, et al. Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg 2010;140:784. [Crossref] [PubMed]
- Easo J, Weigang E, Hölzl PP, et al. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection: Analysis of the German Registry for Acute Aortic Dissection Type A. Ann Cardiothorac Surg 2013;2:175-80. [PubMed]
- Motomura N, Miyata H, Tsukihara H, et al. Risk model of thoracic aortic surgery in 4707 cases from a nationwide single-race population through a web-based data entry system: the first report of 30-day and 30-day operative outcome risk models for thoracic aortic surgery. Circulation 2008;118:S153-9. [Crossref] [PubMed]
- Luo J, Fu X, Zhou Y, et al. Aortic Remodeling Following Sun's Procedure for Acute Type A Aortic Dissection. Med Sci Monit 2017;23:2143-50. [Crossref] [PubMed]
- Ma WG, Zheng J, Liu YM, et al. Dr. Sun's Procedure for Type A Aortic Dissection: Total Arch Replacement Using Tetrafurcate Graft With Stented Elephant Trunk Implantation. Aorta (Stamford) 2013;1:59-64. [Crossref] [PubMed]
- Park SJ, Jeon BB, Kim HJ, et al. Aortic arch repair under moderate hypothermic circulatory arrest with or without antegrade cerebral perfusion based on the extent of repair. J Thorac Dis 2018;10:1875-83. [Crossref] [PubMed]