An update on measurement and monitoring of cough: what are the important study endpoints?
Introduction
Cough is associated with significant physical and psychological morbidity (1). The assessment of cough severity is important for evaluating the response to therapy (2,3). The severity of cough can be measured in several aspects: symptom severity, frequency, intensity and impact on quality of life. A number of validated tools are now available to assess cough (Table 1). A combined subjective and objective assessment is necessary for comprehensive evaluation (4). This review will focus on the measurement and monitoring in adult patients with chronic cough.
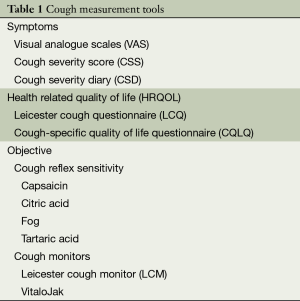
Full table
Visual analogue scales (VAS)
VAS are widely used for the subjective assessment of cough because they are brief and easy to use (5), with which the subject is asked to mark on a 100 mm scale between ‘no cough’ and ‘the worst cough severity’. The advantage of VAS is that they assess the symptom in isolation and reflect the severity. They are also freely available. The VAS is practical for use in research. However, it is still lacks published data reporting its validity and the minimal important difference (MID). The VAS has also been evaluated in acute and sub-acute cough (6,7). The MID has been reported to be 17 mm in acute cough (6). The MID for VAS in chronic cough is likely to be similar to that of acute cough in the authors’ opinion but this has not be studied. The VAS is highly responsive to change (8). In the authors’ opinion, the use of VAS should be encouraged because it is familiar to clinicians, brief and clinically meaningful. Furthermore, similar tools are used effectively in the management of other symptoms such as chest pain. The benefits of using the VAS include improved communication between clinicians regarding the severity of cough and documenting longitudinal observations. The VAS can also be used to assess the urge, frequency and intensity of cough.
Health related quality of life (HRQOL)
Cough can have a wide-ranging impact on the patient, and is very disruptive. It can lead to physical symptoms such as syncope, chest pain, urine incontinence, vomiting, headache, and sleep disturbance.
It is associated with psychological morbidity such as anxiety and depression and socially it can lead to embarrassment and disruption of activities. HRQOL can be quantified by using specifically designed questionnaires. Their advantage in comparison to VAS scales is that they capture the wider impact of cough on the individual. HRQOL questionnaires provide a structured and standardised approach to quantifying health status. They are well validated for this purpose and highly responsive to change (9). HRQOL questionnaires can be categorised into generic tools, such as the Short Form-36 (SF-36) or disease specific. A limitation of generic tools is that they are generally longer questionnaires and potentially less responsive to change. It is advisable to use cough-specific HRQOL questionnaires for the assessment of health status in patients with cough. The two most widely used HRQOL questionnaires for adult patients with chronic cough are the Leicester cough questionnaire (LCQ) and cough-specific quality of life (CQLQ) (10,11). For children, a recently validated questionnaire is now available: the paediatric cough-quality of life questionnaire (PC-QLQ) (12).
Leicester cough questionnaire (LCQ)
The LCQ is a 19-item questionnaire comprising three health domains: physical, psychological and social (10). It is brief, easy to use and score. It was developed using a patient-rated importance scale, also known as a clinimetric method, for patients with chronic cough but has also been validated for patients with chronic obstructive pulmonary disease (COPD), bronchiectasis and acute cough (13-15). It was translated into a wide range of languages and it has been the most widely used of all cough HRQOL questionnaires since 2001. The LCQ is well validated with very good internal reliability, repeatability and responsiveness (10). The MID in acute and chronic cough are 2.0 and 1.3 respectively (6,15). The LCQ has been used in clinical trials of Erythromycin, Gabapentin, cough suppression physiotherapy and Interferon therapy. It is currently being used in a clinical trial of Transient Receptor Potential Ankyroid Receptor Type 1 inhibitor.
Cough-specific quality of life questionnaire (CQLQ)
The CQLQ, developed by clinimetric methodology, is a 28-item questionnaire with 6 domains developed in the US (11). The CQLQ is well validated in chronic and acute cough. It has good internal reliability, repeatability, responsiveness and the MID in chronic cough is 13 units (16). It has recently been used in a clinical trial of Esomeprazole in chronic cough and Thalidomide in patients with cough associated with idiopathic pulmonary fibrosis (17,18).
Other subjective questionnaires
Cough severity score (CSS)
The CSS is a two-part questionnaire referring to symptoms during the day and night time (19). The response scale captures cough frequency, intensity and overall impact. There is little clinical experience with this tool and the MID has not been reported. Further studies of this tool are underway.
Cough severity diary (CSD)
CSD is a brief tool, comprising seven items (20). It was developed using feedback from patients. In addition to severity, it captures the impact of cough intensity. There is, however, little clinical experience with this tool. The MID has not been studied.
Objective assessment of cough
There has been significant progress recently in the development of objective tools to assess cough. The clinical use of objective tools is to validate the presence of cough in subjects and evaluate the improvement following therapy. Until the development of cough monitors, assessment of cough reflex sensitivity was the only objective method being used. The limitation of cough reflex sensitivity measurement is that it only assesses the mechanism of cough, not the efficacy from the patient’s perspective. The recent technological advances in recording devices have led to significant achievements in the field of cough detection monitoring. There is general consensus that the assessment of cough frequency is the gold-standard objective tool (21). It is also possible to measure cough intensity with physiological measures, but they are invasive, and not practical for the clinical setting.
Cough reflex sensitivity—the cough challenge test
The methodology to measure the sensitivity of the cough reflex is, in principle, similar to that used to assess bronchial responsiveness with agents such as methacholine. Cough is provoked by the inhalation of nebulised tussive agents, usually capsaicin or citric acid. Other valid tussive agents include tartaric acid, fog, cinnamaldehyde and bradykinin. The test result is usually expressed as the concentration of tussive agent that causes two or five coughs (C2 or C5). Cough reflex sensitivity assessment is reproducible and responsive in patients (22). It is frequently used in both animal and human research studies. A major limitation to its use in clinical practice is its inability to discriminate patients with cough from healthy subjects (22). Another limitation is that capsaicin dilutions need to be made frequently due to its instability in solution in contrast to other challenge tests such as methacholine. The test also needs to be performed in a ventilated room. The utility of cough reflex sensitivity tests in clinical trials is subsiding because they do not consistently reflect the efficacy of therapy from the patient’s perspective. Its future use might be limited to researchers studying the mechanism of action of anti-tussive therapy. It may be particularly useful in drug development when specific cough reflex pathways can be investigated. The future challenge lies in developing methodology that can reliably discriminate healthy subjects from those with cough, and this may be possible by using higher concentrations of tussive agents than currently used (23).
Cough frequency monitors
Cough frequency assessment is considered the gold standard for the objective assessment of cough. Ten years ago, the development of cough monitors was limited by the recording capacity of tape recorders and poor battery life. The development of MP3 recorders overcame hardware limitations, and therefore the focus turned to the development of software for automated cough detection. There has been significant progress in the development of automated cough detection software, but with mixed results. Many cough monitors have insufficient accuracy for cough detection, and therefore their use is limited or they are not used at all, such as the Hull Automated Cough Monitor, LifeShirt and Pulmotrack (24-26). A particular challenge has been the discrimination of cough sounds from speech and other noise. Two cough monitoring systems have demonstrated good validity and are being used more widely in clinical trials, the Leicester cough monitor (LCM) and the VitaloJak. They differ in their approach to cough detection; the VitaloJak requires manual assessment of condensed cough recordings, and the LCM is largely automated.
VitaloJak
The VitaloJak consists of two microphones (contact and free-field) and an MP3 recorder (27). A condensed version of the recording is assessed manually to listen for cough sounds. The condensed 24-hour cough recording is on average 1.5 hours long. The accuracy of this monitor is dependent on the observer conducting the manual counting and, in experienced hands, it is very good (27). A limitation of manual assessment is that it is labour-intensive and time-consuming. In Table 2, we compare the characteristics of the VitaloJak and LCM. The characteristics are similar, despite the very different approaches to cough detection.
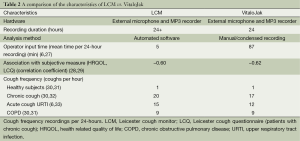
Full table
Leicester cough monitor (LCM)
The LCM comprises of a free-field microphone and an MP3 recorder (34). Cough detection is largely automated using specifically designed software. This involves minor refinement by an operator (5 minutes per 24-hour recording). The sensitivity and specificity for cough detection is very good (30,34). The LCM has been used in single and multi-centre clinical trials (35,36).
The relationship between objective cough frequency and subjective measures of cough
The relationship between objective cough frequency and subjective measures of cough such as VAS and HRQOL is mild to moderate (28). This reflects the different aspects of cough assessed by these tools such as perception vs. actual frequency. The poor relationship does not imply that cough frequency monitoring is inaccurate for the detection of cough. The accuracy of automated cough monitors is established by comparison to manually counted recordings (34).
Cough frequency monitors in the clinical setting
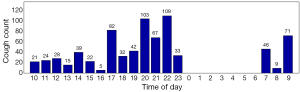
Cough frequency monitors are now sufficiently practical for use in the clinic and clinical trials. The automation of cough monitoring has facilitated this. Patients with chronic cough on average cough every two minutes in a 24-hour period (Figure 1). This contrasts with healthy subjects who cough on average every thirty minutes. Cough has a diurnal pattern in both disease and health states; cough is significantly reduced at night. In patients complaining of cough, the pattern and frequency of cough is very similar irrespective of underlying causes such as gastro-oesophageal reflux and cough variant asthma. In the authors’ experience, the frequency of cough events in patients with chronic lung diseases such as asthma, COPD and idiopathic pulmonary fibrosis depends on the prominence of cough as a symptom. In some patients, the frequency can be as high as those with idiopathic chronic cough. Cough monitors are the best tools to discriminate patients with cough from healthy subjects.
The utility of cough monitor in clinical practice has not been established. The severity of cough can simply be assessed by asking the patient, but the disadvantage of this is that some patients and clinicians may be poor judges of symptom severity. Cough monitors can potentially be used to validate the presence of cough. They can also be used to quantify the response to therapy. They have been used in the research setting to assess the temporal relationship of cough with episodes of gastro-oesophageal reflux; the clinical usefulness of this has not been established (37). The benefits of cough monitoring technology in the clinic need further investigation. Cough monitors however are considered an important end-point in clinical trials. The strength of cough monitors is their objectivity and that they can discriminate healthy subjects from those without cough. This may be useful for selecting patients for clinical trials. Cough frequency has been reported to be a repeatable measure in patients with stable chronic cough. In contrast, in acute cough, cough frequency is not repeatable due to natural recovery (6). The rate of improvement in cough frequency may be a better measure in such patients.
Cough frequency monitors as a study endpoint
Cough frequency can be expressed by a range of outcome measures. For example, absolute counts vs. coughs per hour. Cough can be assessed during daytime, night time or 24 hours. It is perhaps better to express change in cough frequency as a percentage or fold change, rather than absolute change, since it has a wide range. An advantage of using cough frequency measures to determine the sample size of studies is that considerably fewer subjects are required in comparison to subjective outcome measures, such as HRQOL. This is due to the comparatively larger change in cough frequency required to demonstrate a MID (6). For example, in acute cough, the MID for cough frequency has been reported as a 54% reduction (6).
Cough intensity
The intensity of cough may also be relevant to the impact on an individual, in addition to the frequency. Little is known about cough intensity and its importance to patients. It can be assessed subjectively with VAS or objectively with physiological measures such as cough flow, oesophageal pressure or electromyography. Flow is the most practical physiological measure since it is non-invasive and relatively easy to perform. However, its limitation is that it is not ideal for continuous monitoring in an ambulatory setting. It may be possible to assess cough intensity with sound (38). Further studies are needed to determine whether cough sound is a valid measure of cough intensity, its relationship with subjective measures and clinical relevance.
Conclusions
A number of tools are now available to assess cough. It is likely that a combination of subjective and objective assessment is necessary to assess cough comprehensively since each tool assesses very different aspects. For subjective assessment, the VAS is ideal for use in the clinic since it is practical, and it can be used to communicate the severity of cough to other clinicians and for longitudinal observation. It is also good for use in clinical trials. The VAS should be complemented by HRQOL assessment to assess impact. HRQOL questionnaires for cough are the most validated of all cough assessment tools. The LCQ and the CQLQ are the most widely used for adult patients with chronic cough. It is arguable that the primary outcome measure of clinical trials should be objective, and cough frequency monitors are best placed for this. Cough frequency monitors are increasingly being used in clinical trials. The clinical experience of using cough monitors to date is that they are practical and valid. They should always be complemented by assessment of HRQOL since a reduction in objective cough frequency without subjective improvement would not be considered clinically important. Further work is necessary in a number of areas to improve the utility of cough assessment tools. The investigation of the MID and more precise sample size estimations are good examples. The assessment of cough in chronic lung disease such as COPD and idiopathic pulmonary fibrosis is likely to be very fruitful.
Acknowledgements
Arietta Spinou is funded by the Greek State Scholarships Foundation (IKY).
Disclosure: Dr. Birring is a developer of cough monitoring and quality of life tools.
References
- Brignall K, Jayaraman B, Birring SS. Quality of life and psychosocial aspects of cough. Lung 2008;186 Suppl 1:S55-8. [PubMed]
- Birring SS. New concepts in the management of chronic cough. Pulm Pharmacol Ther 2011;24:334-8. [PubMed]
- Birring SS. Developing antitussives: the ideal clinical trial. Pulm Pharmacol Ther 2009;22:155-8. [PubMed]
- Birring SS. Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med 2011;183:708-15. [PubMed]
- Birring SS, Parker D, Brightling CE, et al. Induced sputum inflammatory mediator concentrations in chronic cough. Am J Respir Crit Care Med 2004;169:15-9. [PubMed]
- Lee KK, Matos S, Evans DH, et al. A longitudinal assessment of acute cough. Am J Respir Crit Care Med 2013;187:991-7. [PubMed]
- Wang K, Birring SS, Taylor K, et al. Montelukast for postinfectious cough in adults: a double-blind randomised placebo-controlled trial. Lancet Respir Med 2014;2:35-43. [PubMed]
- Birring SS, Passant C, Patel RB, et al. Chronic tonsillar enlargement and cough: preliminary evidence of a novel and treatable cause of chronic cough. Eur Respir J 2004;23:199-201. [PubMed]
- Patel AS, Watkin G, Willig B, et al. Improvement in health status following cough-suppression physiotherapy for patients with chronic cough. Chron Respir Dis 2011;8:253-8. [PubMed]
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [PubMed]
- French CT, Irwin RS, Fletcher KE, et al. Evaluation of a cough-specific quality-of-life questionnaire. Chest 2002;121:1123-31. [PubMed]
- Newcombe PA, Sheffield JK, Juniper EF, et al. Validation of a parent-proxy quality of life questionnaire for paediatric chronic cough (PC-QOL). Thorax 2010;65:819-23. [PubMed]
- Berkhof FF, Boom LN, ten Hertog NE, et al. The Validity and Precision of the Leicester Cough Questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes 2012;10:4. [PubMed]
- Murray MP, Turnbull K, MacQuarrie S, et al. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J 2009;34:125-31. [PubMed]
- Yousaf N, Lee KK, Jayaraman B, et al. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough 2011;7:4. [PubMed]
- Fletcher KE, French CT, Irwin RS, et al. A prospective global measure, the Punum Ladder, provides more valid assessments of quality of life than a retrospective transition measure. J Clin Epidemiol 2010;63:1123-31. [PubMed]
- Shaheen NJ, Crockett SD, Bright SD, et al. Randomised clinical trial: high-dose acid suppression for chronic cough - a double-blind, placebo-controlled study. Aliment Pharmacol Ther 2011;33:225-34. [PubMed]
- Horton MR, Santopietro V, Mathew L, et al. Thalidomide for the treatment of cough in idiopathic pulmonary fibrosis: a randomized trial. Ann Intern Med 2012;157:398-406. [PubMed]
- Hsu JY, Stone RA, Logan-Sinclair RB, et al. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. Eur Respir J 1994;7:1246-53. [PubMed]
- Vernon M, Kline Leidy N, Nacson A, et al. Measuring cough severity: development and pilot testing of a new seven-item cough severity patient-reported outcome measure. Ther Adv Respir Dis 2010;4:199-208. [PubMed]
- Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J 2007;29:1256-76. [PubMed]
- Prudon B, Birring SS, Vara DD, et al. Cough and glottic-stop reflex sensitivity in health and disease. Chest 2005;127:550-7. [PubMed]
- Hilton EC, Baverel PG, Woodcock A, et al. Pharmacodynamic modeling of cough responses to capsaicin inhalation calls into question the utility of the C5 end point. J Allergy Clin Immunol 2013;132:847-55. [PubMed]
- Barry SJ, Dane AD, Morice AH, et al. The automatic recognition and counting of cough. Cough 2006;2:8. [PubMed]
- Coyle MA, Keenan DB, Henderson LS, et al. Evaluation of an ambulatory system for the quantification of cough frequency in patients with chronic obstructive pulmonary disease. Cough 2005;1:3. [PubMed]
- Vizel E, Yigla M, Goryachev Y, et al. Validation of an ambulatory cough detection and counting application using voluntary cough under different conditions. Cough 2010;6:3. [PubMed]
- Barton A, Gaydecki P, Holt K, et al. Data reduction for cough studies using distribution of audio frequency content. Cough 2012;8:12. [PubMed]
- Birring SS, Matos S, Patel RB, et al. Cough frequency, cough sensitivity and health status in patients with chronic cough. Respir Med 2006;100:1105-9. [PubMed]
- Decalmer SC, Webster D, Kelsall AA, et al. Chronic cough: how do cough reflex sensitivity and subjective assessments correlate with objective cough counts during ambulatory monitoring? Thorax 2007;62:329-34. [PubMed]
- Yousaf N, Monteiro W, Matos S, et al. Cough frequency in health and disease. Eur Respir J 2013;41:241-3. [PubMed]
- Sumner H, Woodcock A, Kolsum U, et al. Predictors of objective cough frequency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:943-9. [PubMed]
- Kelsall A, Decalmer S, McGuinness K, et al. Sex differences and predictors of objective cough frequency in chronic cough. Thorax 2009;64:393-8. [PubMed]
- Sunger K, Powley W, Kelsall A, et al. Objective measurement of cough in otherwise healthy volunteers with acute cough. Eur Respir J 2013;41:277-84. [PubMed]
- Birring SS, Fleming T, Matos S, et al. The Leicester Cough Monitor: preliminary validation of an automated cough detection system in chronic cough. Eur Respir J 2008;31:1013-8. [PubMed]
- Yousaf N, Monteiro W, Parker D, et al. Long-term low-dose erythromycin in patients with unexplained chronic cough: a double-blind placebo controlled trial. Thorax 2010;65:1107-10. [PubMed]
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:1583-9. [PubMed]
- Decalmer S, Stovold R, Houghton LA, et al. Chronic cough: relationship between microaspiration, gastroesophageal reflux, and cough frequency. Chest 2012;142:958-64. [PubMed]
- Pavesi L, Subburaj S, Porter-Shaw K. Application and validation of a computerized cough acquisition system for objective monitoring of acute cough: a meta-analysis. Chest 2001;120:1121-8. [PubMed]