|
Original Article
Establishment of an orthotopic lung cancer model in nude mice and its evaluation by spiral CT
1Southern Medical University; 2Guangzhou Institute of Respiratory Disease, State Key Laboratary of Respiratory Disease; 3Department of Cardiothoracic Surgery; 4Department of Radiology, The First Hospital Affiliated to Guangzhou Medical College, Guangzhou 510120, China
|
|
Abstract
Objective: To establish a simple and highly efficient orthotopic animal model of lung cancer cell line A549 and evaluate the growth pattern of intrathoracic tumors by spiral CT. Methods: A549 cells (5×106mL-1) were suspended and inoculated into the right lung of BALB/c nude mice via intrathoracic injection. Nude mice were scanned three times each week by spiral CT after inoculation of lung cancer cell line A549. The survival time and body weight of nude mice as well as tumor invasion and metastasis were examined. Tissue was collected for subsequent histological assay after autopsia of mice. Results: The tumor-forming rate of the orthotopic lung cancer model was 90%. The median survival time was 30.7 (range, 20-41) days. The incidence of tumor metastasis was 100%. The mean tumor diameter and the average CT value gradually increased in a time-dependent manner. Conclusions: The method of establishing the orthotopic lung cancer model through transplanting A549 cells into the lung of nude mice is simple and highly successful. Spiral CT can be used to evaluate intrathoracic tumor growth in nude mice vividly and dynamically. Key words
Lung cancer; orthotopic lung cancer model; spiral CT; A549
J Thorac Dis 2012;4(2):141-145. DOI: 10.3978/j.issn.2072-1439.2012.03.04
|
|
Introduction
Lung cancer is by far one of the malignant tumors with the highest incidence and mortality all over the world. Lung cancer can be assigned into small cell and non-small cell lung cancer, among which the latter accounts for 85% with a 5-year survival rate of only about 15% (1). Over the past few decades, research on lung cancer has made great progress, but its response rate and survival improvement have been very slow. Selecting a simple and feasible tumor-bearing animal model is one of the important ways to study the pathogenesis and curative effect of lung cancer. The ectopic subcutaneous tumor-bearing animal model of lung cancer is convenient to be established and conducive to observation of tumor growth. It has been extensively applied for the drug sensitivity assay of lung cancer (2). Nonetheless, the ectopic subcutaneous tumor-bearing animal model does not represent the real tumor in real environment, since the growth and dissemination of tumor cells in vivo are organ-specific (3). Compared with the tumor model constructed by ectopic subcutaneous inoculation, orthotopic lung cancer can mimic natural environment of tumorigenesis (3). Thus, it is of necessity to establish an appropriate orthotopic animal model of non-small cell lung cancer, to observe the characteristics of tumor growth and evaluate its efficacy.
Many researchers have constructed orthotopic tumor models of urologic neoplasms and digestive tract tumors in immunodeficient SCID mice and nude mice making use of corresponding malignant tumor cells (4,5). McLemore et al. was the first establishing an orthotopic model of human lung cancer in nude mice through endobronchial injection (6). Later researchers adopted injection of tumor cells via the tail vein (7,8) and intrapulmonary inoculation of the tumor mass (9). However, the abovementioned methods have certain limitations because
of great technical difficulties, which require the experiment
operator to have considerable proficiency. In addition, their
success rates are not high. This study aims at building up an
orthotopic animal model of lung cancer in BALB/c nude mice
by intrapulmonary inoculation of cancer cell suspension, so as to
learn intrapulmonary tumor growth and changes of nude mice
coupled with dynamic spiral CT through observation of the
survival time and status of treated mice.
|
|
Methods and materials
Cells and cell cultivation
Lung adenocarcinoma cell line A549 was obtained from the
Shanghai Institute of Cell Biology of the Chinese Academy of
Sciences, which was incubated using DMEM medium containing
10% calf serum (US Hyclone Cooperation, Logan, US) (serum
derived from Brazil and manufactured in Logan, Utah, US, article
no. sv30087.01, batch no. NvM0347) in a 5% CO2 incubator at
37 ℃ and routinely sub-cultured.
Animal and animal feeding
A total of 20 male nude BALB/c nu/nu mice aged 6-8 weeks
and weighing 20-22 g were purchased from the Guangdong
Provincial Medical Experimental Animal Center, the certificate
no. of qualified animals SCXK (Yue) 2008-0002, raised in SPF
environment at room temperature (25+2) ℃ and given aseptic
full-price nutritional pellet feed and sterile water.
|
|
Methods
A total of 20 nude BALB/c nu/nu mice were intraperitoneally
injected with pentobarbital sodium (10 mg/kg) to induce
anesthesia and fixed in the right lateral decubitus position after
anesthesia. Then 100 μL A549 single cell suspension (5×106 mL-1)
prepared with the 1 mL injector was percutaneously inoculated
into the upper margin of the sixth intercostal rib on the right
anterior axillary line to a depth of about 5 mm rapidly and after
that, the needle was promptly pulled out. Nude mice were
maintained in the right lateral decubitus position after injection
and observed until complete recovery.
Spiral CT scanning
Mice were fixed on a plane plate in a supine position following
intraperitoneal injection of pentobarbital sodium (10 mg/kg) to
induce anesthesia. The Toshiba Aquilion16-slice CT scanner was
adopted to perform routine thin-slice plain CT scan from the mouse
neck to abdomen, slice thickness 1mm, reconstruction interval 0.5-0.8 mm, tube voltage 100 kV and tube current 90-110 mA.
Survival index detection
Body weight
Body weight of each mouse was measured using the electronic
balance every 4 days (precision: 0.1 g) and data were stored in
EXCEL for inspection.
Survival time
Survival time was the time from modeling to natural death of
mice.
Gross observation
Lung cancer cell invasion and metastasis in thoracic, mediastinal
and pericardial parts as well as pleural effusion of mice were
observed in the operation group.
Tissue embedding and pathological detection
After anesthesia, mice were sacrificed by cutting off the neck
under anesthesia to remove the intrathoracic heart, bilateral
lungs, pleura, lymph nodes and mass etc, which were fixed in
10% formalin solution and embedded in paraffin to make into
tissue sections, subjected to HE staining and observed under a
microscope.
Data analysis
Data were processed using SPSS 13.0 software package and
expressed as
|
|
Results
Survival index results of nude mice
Intrathoracic implantation via puncture succeeded in all the
20 nude mice. Gross anatomy and pathological confirmation
following termination of experimental observation showed that
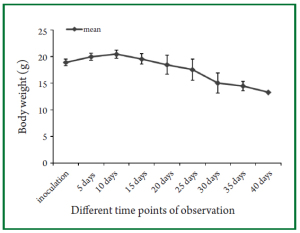
the success rate of tumor implantation was 90% (18/20). Figure 1
shows the trend in mean body weight changes at different time
points of observation after inoculation of tumor cells. It can be
seen that the mean body weight of nude mice continually declined
at around 10 days following intrathoracic inoculation of tumor
cells. At around two weeks following inoculation of tumor cells,
tumor-bearing nude mice developed similar cachectic symptoms
as observed in clinical patients with tumors and symptoms of
labored mouth breathing could be observed in dying nude mice.
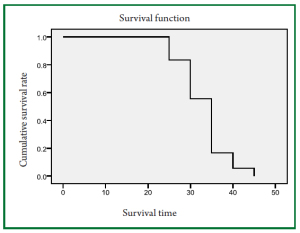
Figure 2 shows the survival curves of nude mice and the median
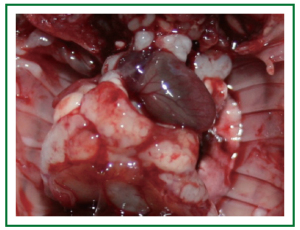
survival time is 30.7 (range, 20-41) days. Figure 3 shows the
image of the gross specimen of tumor-bearing nude mice, within
which the right lung mass is clearly visible and there are visible metastatic foci in contralateral lung tissue, mediastinal tissue and
chest wall. The intrathoracic tumor metastasis rate of nude mice
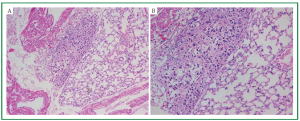
was 100% (18/18). Figure 4 is the diagram for HE staining of
histopathological sections of lung tissue in nude mice. Figure 4A
indicates that tumor tissue is located in the central region of the
visual field, which developed accompanying alveolar tissue and
blood vessels. Figure 4B indicates irregular tumor cell alignment,
nuclear hyperchromatism and obvious heteromorphism after
magnifying.
Figure 4. Microscopic features of the mouse lung. H/E stain of the paraffin-embedded sections of tumor developed within the mouse lung.
(A ×100; B ×200).
Observation of orthotopically implanted tumor growth in nude
mice using spiral CT
Spiral CT scan was performed at day 7, 14 and 21 on tumorbearing
nude mice after intrathoracic injection of lung tumor cell
suspension into the right chest for three consecutive weeks, to observe intrathoracic tumor growth in nude mice dynamically.
Intrathoracic tumor diameters for the three times of spiral CT
scanning were respectively: (1.1±0.4) , (2.7±0.7) and (4.8±1.4) mm.
Intrathoracic tumor CT values for the three times of spiral CT
scanning were respectively (32±12) , (68±15) and (89±13)
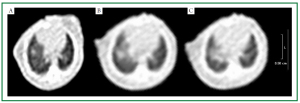
HU. Figure 5 shows the results of spiral CT scanning of the
orthotopically implanted tumor of lung cancer for the same
nude mouse. Figure 5A shows small right lung nodules one
week after intrathoracic injection of tumor cell suspension
and gradual growth of intrathoracic tumor mass over time.
Figure 5C shows right lung mass enlarged for several times and
widened mediastinal shadow at week 3, which was confirmed as
mediastinal metastasis by gross anatomy.
Figure 5. CT image of the orthotopic xenograft of lung cancer in nude mice. Figure A shows small right lung nodules one week after intrathoracic injection of
tumor cell suspension and Figure B shows gradual growth of intrathoracic nodules; Figure C shows right lung mass markedly enlarged for several times.
|
|
Discussion
Our studies have led to the development of a rapid and reproducible
model of orthotopic lung cancer in which human lung tumorderived
cell lines can be engrafted throughout the pulmonary
parenchyma. Among the several orthotopic lung cancer modeling
methods commonly seen by far, the tumor-forming rate of injection
via the tail vein is as high as 90-100%, but this method may lead to
pulmonary vascular embolism by tumor cells thus causing death
(7,8); intratracheal injection is intractable and its tumor-forming
rate is only around 80% (10,11). In this study, direct intrathoracic
injection was applied to establish the orthotopic lung cancer animal
model using common human lung adenocarcinoma cell line
A549 and the tumor-forming rate of modeling was 90%, similar
to those obtained using other lung cancer cell lines (2,12,13). An
advantage of this model includes the simple and easy implantation
procedure by direct puncturing through the intercostal space to lung
parenchyma, without thoracotomy or intubation.
In this study, spiral CT was also adopted to perform realtime
evaluation of tumor formation following intrathoracic inoculation of nude mice, showing solid tumor formation in
right chest of mice at around one week following injection
of tumor cell suspension. The trend of body weight changes
in tumor-bearing nude mice in Figure 1 reflected a declining
trend of mean body weight of tumor-bearing nude mice at
around 10 days after inoculation of tumor cells and the time
point for body weight decline of tumor-bearing nude mice
was basically consistent with the emergence time of tumor
formation indicated in CT results. Thus, in this model, it is
critical to observe body weight changes in nude mice following
orthotopic injection of tumor cells to judge the successfulness
of tumor formation. Gross anatomy and pathological detection
of nude mice successfully established with an orthotopic lung
cancer model confirmed metastatic foci in contralateral lung
tissue, mediastinal tissue and chest wall to varying degrees,
with an intrathoracic tumor metastasis rate of 100%, consistent
to other similar reports (2,13). This may not only be related to
characteristics of tumor cell metastasis and invasion, but also
be related to the short survival time of nude mice following
tumor formation and short observation time.
In the preliminary experiment of this study, two nude mice
rapidly died of intrathoracic massive hemorrhage and respiratory
tract symptoms of hemoptysis after withdrawal of the puncture
needle. This can mainly be explained as follows: the point of
puncture was located besides the posterior axillary line of the sixth
intercostal space of right chest wall, which was close to the hilar
vessels after insertion of the needle and could pierce into hilar
vessels to induce intrathoracic massive hemorrhage and rapid
death, as also confirmed by subsequent gross anatomy. In order to
avoid injury to hilar vessels, the location adjacent to the median
or anterior axillary line of the sixth intercostal space was chosen as
the point of puncture and the depth of needle insertion was strictly controlled at around 5 mm. The subsequent puncture process was
successful without occurrence of aforementioned phenomena.
The orthotopic animal model of lung cancer can well mimic
the real environment of tumor growth, but due to tumor location
within the chest, it is not conducive to real-time observation of
the implanted tumor mass in the experimental process. In some
orthotopic lung cancer model studies, pathological anatomy
is performed after execution of a certain number of nude mice
regularly or at the end point of observation to monitor tumor growth
and dissemination, but this method requires a big number of nude
mice and a great deal of time or energy (12,13). In this experiment,
spiral CT scan was employed to conduct dynamic observation
of intrapulmonary tumor growth within mice, which can not
only clearly observe the successfulness of intrathoracic tumor
implantation, but also observe the gradual tumor growth in vivo over
time, to judge the occurrence of mediastinal tumor metastasis. Some
studies also put forward using micro-MRI to assess the lung tumor
model in small animals (14), but some scholars have pointed out
that among lung tumor images, compared to micro-MRI, micro-
CT behaves better in contrast imaging of air and soft tissue (15),
so more studies apply micro-CT to observe the lung cancer model
of rodents (15-17). Indeed, micro-CT is superior to conventional
spiral CT in resolution of in vivo imaging of small animals, but
micro-CT equipment is not liable to be obtained for many research
units and ordinary spiral CT can achieve the slice thickness of
1mm, which can observe orthotopic intrathoracic tumor growth
and development of nude mice. Thus, as an objective and feasible
method, dynamic spiral CT scanning can be further used for efficacy
observation during antitumor drug experiments.
To sum up, construction of orthotopic human lung cancer
model via direct intrathoracic injection of human non-small
cell lung cancer cell suspension is convenient, has a high
tumor-forming rate and can better mimic the occurrence and
development of clinical non-small cell lung cancer. Spiral CT
scanning can perform dynamic observation and evaluation of the
whole process of modeling.
|
|
References
Cite this article as: Liu X, Liu J, Guan Y, Li H, Huang L, Tang H, He J.
Establishment of an orthotopic lung cancer model in nude mice and its
evaluation by spiral CT. J Thorac Dis 2012;4(2):141-145. doi: 10.3978/
j.issn.2072-1439.2012.03.04
|