
Prognostic factors in stage IB non-small cell lung cancer according to the 8th edition of the TNM staging system after curative resection
Introduction
In 2015, the International Association for the Study of Lung cancer (IASLC) proposed the 8th edition of the tumor, node, and metastasis (TNM) classification of lung cancer, and the new staging system enacted in January 2017 (1). The new staging system contained modifications in stage groupings, but stage IB non-small cell lung cancer (NSCLC) had not been changed. According to the previous edition of the TNM staging system, only T2aN0M0 was considered stage IB disease. Stage IB of the 8th edition TNM classification also only includes T2aN0M0 (2,3). However, some criteria for T classifications were changed (1,4,5). First, the measurement of tumor size was changed. Previously, the tumor size was determined by the maximum size of the tumor including lepidic component. On the other hand, the T stage in the 8th edition is determined according to the maximum size of the invasive component, without the lepidic component. Second, the T2a category also changed. In the 7th edition, T2a was considered to be a tumor size ranging from 3 to 5 cm; whereas in the 8th edition, the T2a descriptor was changed to a size of the invasive component ranging from 3 to 4 cm. Therefore stage migration also occurred for stage IB NSCLC after adoption of the 8th edition of TNM staging system.
According to the National Comprehensive Cancer Network (NCCN) guidelines for NSCLC (Version 3.2019), adjuvant treatment after surgery may be considered for patients with several risk factors. In the NCCN guidelines, risk factors include poorly differentiated tumors, lymphovascular invasion, wedge resection, tumors >4 cm, visceral pleural invasion, and unknown lymph node status. These risk factors have been reported by several studies to be prognostic factors in patients with early-stage lung cancer (6-9). However, a re-evaluation on whether or not the prognostic factors for stage IB NSCLC remain the same for the new staging system is needed. In fact, no study has evaluated the prognostic factors of stage IB disease as classified by the new 8th edition of the TNM staging system. In addition, since stage classifications have changed, the efficacy of adjuvant chemotherapy for stage IB NSCLC also must be re-evaluated.
The aim of this study was to identify the prognostic risk factors in patients with stage IB NSCLC according to the 8th edition of TNM classification and to determine the outcomes of patients with stage IB NSCLC who received adjuvant chemotherapy.
Methods
Patients
From 2005 to 2016, 1,598 consecutive patients at a tertiary hospital in South Korea were diagnosed with NSCLC and underwent curative surgery. Of this population, 262 patients were diagnosed with 8th edition stage IB NSCLC. Finally, 211 patients satisfied the following study criteria: (I) anatomical lobectomy or bilobectomy; (II) complete resection with clear resection margins; (III) no neoadjuvant treatment. The clinicopathological characteristics of all study patients were analyzed. We compared the clinicopathological characteristics and survival between the patients undergoing surgery only versus those who also received adjuvant chemotherapy. The risk factors for recurrence and cancer-related death were analyzed.
Surgical procedures
The operative procedures included lobectomy and bilobectomy. The surgery also included complete dissection of N1 lymph nodes. Most patients underwent mediastinal lymph node dissection. Systematic nodal dissection was defined as mediastinal lymph node dissection of more than 3 mediastinal lymph node stations and included the subcarinal lymph nodes. Selective mediastinal node dissection was defined as mediastinal lymph node dissection near the involved lobe.
Adjuvant chemotherapy
We defined adjuvant chemotherapy as postoperative chemotherapy involving the use of platinum-based agents. The selection of chemotherapy regimens depended on clinician preference. Most regimens included carboplatin or cisplatin combined with paclitaxel or vinorelbine. Chemotherapy was administered 1 month after surgery. Four cycles of chemotherapy were routinely recommended. There were no definite indications for adjuvant chemotherapy. When tumor size was larger than 4 cm or lymphovascular invasion was present, a clinician sometimes recommended adjuvant chemotherapy. The final decision was made by the patient.
Histological evaluation and restaging to the 8th-edition staging system
All clinical specimens and pathology reports were reviewed. Pathology report included tumor size, tumor location, nodal status, and pleural or lymphovascular invasion. Visceral pleural invasion was defined as tumor extending beyond the elastic layer. Lymphovascular invasion was defined as tumor cells present in lymphatic or vascular lumina. TNM staging was based on the 8th edition of the TNM staging system of lung cancer (2). To reclassify the T category according to the 8th edition, tumor size was remeasured by the pathologist at the greatest diameter of the invasive component on a histopathological preparation (5).
Statistical analysis
The clinical and pathological characteristics of the surgery-only patients and adjuvant-chemotherapy patients were compared. The Student t-test or Wilcoxon rank-sum test was used for continuous variables, and the χ2 test or Fisher exact test was applied for categorical variables.
The Kaplan-Meier method was used to analyse data collected from the interval between the time of operation and the time of the final follow-up visit. Recurrence-free-survival (RFS) rates and disease-specific-survival (DSS) rates were estimated by the Kaplan-Meier method using data collected from patients with confirmed recurrence and cancer-related death. The Cox proportional hazards model was used in a multivariate analysis to determine the risk factor of recurrence and cancer-related death for all the study patients. The variables with a P value <0.1 by the univariate analysis were entered into a multivariate analysis. A P value <0.05 was considered statistically significant.
Results
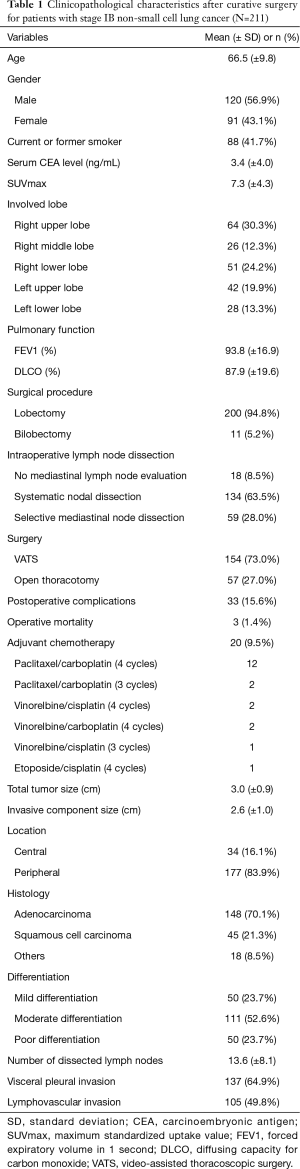
Table 1 shows the clinicopathological characteristics of patients with stage IB NSCLC. Lobectomy was performed for 200 patients (94.8%) and bilobectomy was performed for 11 patients (5.2%). Most patients (91.5%) underwent mediastinal lymph node dissection. Systematic nodal dissection was conducted in 134 patients (63.5%) and selective mediastinal node dissection in 59 patients (28.0%). Postoperative complications occurred in 33 patients (15.6%), and 3 patients (1.4%) died after surgery. Adjuvant chemotherapy was administered to 20 patients after surgery. Histopathological analysis revealed a mean total tumor size (including the lepidic component) of 3.0 cm. The mean invasive component size (excluding the lepidic component) was 2.6 cm. The most common histological tumor type was adenocarcinoma (70.1%). Visceral pleural invasion and lymphovascular invasion were present in 137 (64.9%) and 105 (49.8%) patients, respectively.
Full table
Comparisons of survival rates of surgery-only patients and adjuvant-chemotherapy patients
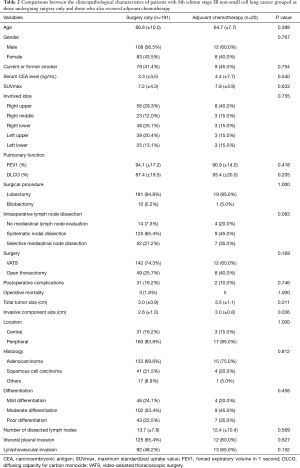
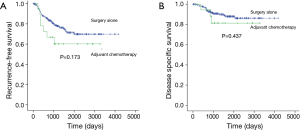
The median follow-up time of the study patients with stage IB NSCLC after surgery was 1,457 days (12–4,172 days). The wide range of the follow-up periods was accounted for by a single patient who died of a postoperative complication 12 days after surgery. Several patients had short follow-up periods, mostly because of cancer-related death or postoperative mortality. We compared the survival rates of patients undergoing surgery only (surgery-only patients, n=191) and of patients receiving adjuvant chemotherapy after undergoing surgery (adjuvant-chemotherapy patients, n=20). The differences between most clinicopathological characteristics in the two groups were not significant (Table 2). Only tumor size and invasive component size were larger in the adjuvant-chemotherapy patients. Recurrence was identified in 53 patients, and the differences between the sites of recurrence in the 2 groups were not significant (Table 3). The 5-year RFS rates of the surgery-only patients and adjuvant-chemotherapy patients were 71.4% and 60.2%, respectively (P=0.173, Figure 1A). The 5-year DSS rates of the surgery-only patients and adjuvant-chemotherapy patients were 88.0% and 81.4%, respectively (P=0.437, Figure 1B).
Full table

Full table
Prognostic factors in stage IB NSCLC according to the 8th edition of the TNM staging system
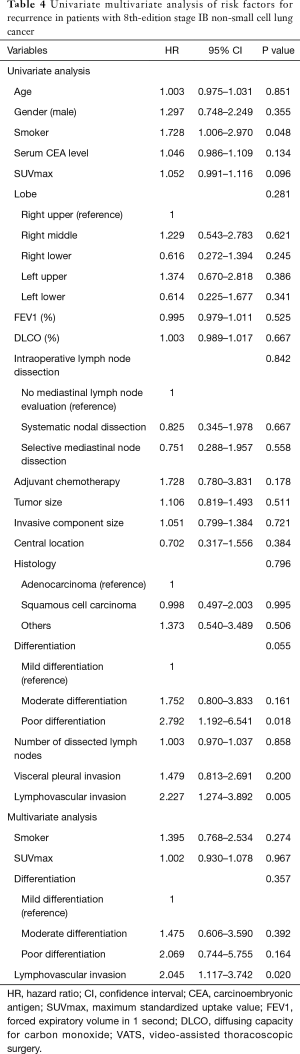
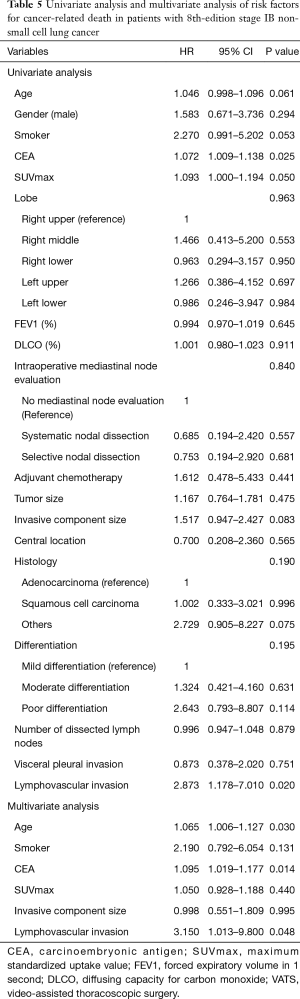
For all the study patients, the 5-year RFS and DSS rates were 70.3% and 87.3%, respectively. We analyzed risk factors for recurrence and cancer-related death. First, univariate and multivariate analyses were conducted to identify the risk factors for recurrence (Table 4). The specific variables identified as significant (P<0.1) by univariate analysis included smoking status, maximum standardized uptake value (SUVmax) on positron emission tomography (PET), tumor differentiation, and lymphovascular invasion. These variables were entered into the multivariate analysis. Multivariate analysis identified lymphovascular invasion as a significant independent risk factor [hazard ratio (HR) =2.045, P=0.020] for recurrence.
Full table
Univariate and multivariate analyses were also conducted to identify the risk factors for cancer-related death (Table 5). The specific variables identified as significant (P<0.1) by univariate analysis included age, smoking status, serum carcinoembryonic antigen (CEA) level, SUVmax, size of invasive component, and lymphovascular invasion. These variables were entered into the multivariate analysis. By multivariate analysis, lymphovascular invasion was the only significant independent risk factor (HR =3.150, P=0.048) for cancer-related death.
Full table
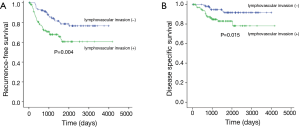
Since the 2 analyses identified lymphovascular invasion as a prognostic factor, we compared the 5-year RFS and DSS rates of patients with tumors that were with or without lymphovascular invasion. The 5-year RFS rates of patients with tumors that were with or without lymphovascular invasion were 60.6% and 78.6%, respectively (Figure 2A, P=0.004). The 5-year DSS rates of patients with tumors that were with or without lymphovascular invasion were 83.0% and 91.7%, respectively (Figure 2B, P=0.015).
Discussion
After implementation of the 8th revision of the TNM classification of NSCLC, the composition of the tumors included in the stage IB classification was changed. Most importantly, the requirement for measuring tumor size was changed. Determination of the T stage in the 8th revision is based only on the maximum dimension of the invasive component and excludes the lepidic component (4,5). The size range of the T2a descriptor was also reduced from 3–5 to 3–4 cm. Therefore, the tumor characteristics for 7th edition stage IB NSCLC were changed in the 8th edition. Because of these changes, we thought that prognostic factors should be re-evaluated for tumors staged IB according to the new staging system.
This study identified lymphovascular invasion in tumors considered to be stage IB NSCLC by the 8th edition of the TNM classification as the only significant prognostic factor. Tumor differentiation, visceral pleural invasion, and tumors measuring 4 cm, which had been considered to be prognostic factors, were not identified as prognostic factors in this study. With regard to tumor size, tumors larger than 4 cm were no longer a risk factor, because only the tumors 4 cm and smaller were considered to be stage IB disease by the 8th edition of the TNM staging system. Other factors warrant re-evaluation in a future larger study.
The surgical resections in this study included more than lobectomy only. When we performed anatomical lobectomies, the N1 lymph nodes were always completely dissected. However, mediastinal lymph node dissection was not performed in all patients, and a few patients did not undergo mediastinal lymph node dissection because of technical difficulties (severe whole lung adhesion or unstable status of patient during operation). Systematic nodal dissection was conducted in 63.5% of the study patients. Indeed, systematic nodal dissection is recommended when lobectomies are performed for lung cancer (10,11), because occult lymph node metastasis can be present even in clinical stage N0 disease (12-14). Systematic nodal dissection is mandatory, especially for stage IB disease, because the possibility of occult lymph node metastasis is higher in stage IB than in stage IA disease (15). Nevertheless, intraoperative mediastinal lymph node dissection was not found to be a prognostic factor in this study. This result is accounted for by the fact that mediastinal lymph node dissection was performed for a large number of patients, and every patient underwent lobectomy to remove the N1 lymph nodes completely. In addition, because skip N2 metastasis rarely occurs without the presence of N1 metastasis, we decided that nodal staging was appropriate in this study.
Adjuvant treatment after surgery is not routinely recommended for stage IB NSCLC (9). However, the prognosis of these patients after undergoing surgical treatment for stage IB disease is not as satisfactory as the prognosis of IA patients after surgery. In this study, the 5-year RFS rate of patients with stage IB NSCLC was 70%. Therefore, additional treatment might be needed after surgery for some cases of stage IB NSCLC. In this study, however, adjuvant chemotherapy did not improve the prognosis of stage IB NSCLC. The differences between the RFS and DSS rates of the surgery-only patients versus adjuvant-chemotherapy patients were not significant. Adjuvant chemotherapy was also not a prognostic factor by multivariate analysis. Therefore, the administration of adjuvant chemotherapy to all patients with stage IB NSCLC is unreasonable, and the outcomes of high-risk patients with stage IB NSCLC who receive adjuvant chemotherapy must be further evaluated. We found that lymphovascular invasion was the independent risk factor for outcome in patients with IB NSCLC as staged by the new 8th edition staging system. We hope to perform prospective randomized controlled trials of adjuvant chemotherapy after surgery for patients with stage IB NSCLC showing lymphovascular invasion.
This study has several limitations. First, it was a retrospective study. Second, we obtained data from a single institution with a relatively small sample size, so generalizing our results is difficult. However, the study patients were treated by a standardized surgical protocol at single institution. Furthermore, a detailed analysis was possible because of detailed data that was stored in the electronic medical records. We also used detailed data from pathological specimens and pathological reports. We believe that our data can be used as the basis for future studies. Since there were only 20 patients undergoing adjuvant chemotherapy, comparing outcomes between the surgery-only and the adjuvant-chemotherapy patients is difficult. However, the main purpose of this study was not to compare the outcomes between patients with and without adjuvant chemotherapy, but to identify prognostic factors in the newly revised stage IB NSCLC. Finally, the follow-up period was relatively short. However, most patients with NSCLC are known to have disease recurrence within a 2-year postoperative period (16), and early recurrence has been shown to be an accurate reflection of long-term outcome (17).
In conclusion, lymphovascular invasion was an independent prognostic factor for patients with stage IB NSCLC after surgical resection. Adjuvant chemotherapy was not an effective treatment for stage IB patients according to the new staging system. Additional studies that include prospective randomized controlled trials might provide more accurate results. Furthermore, the efficacy of adjuvant chemotherapy for stage IB NSCLC with lymphovascular disease should be evaluated.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board of Seoul St. Mary’s Hospital at the Catholic University of Korea and individual consent was waived (Referral number: KC19RESI0158).
References
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Yang HC, Kim HR, Jheon S, et al. Recurrence Risk-Scoring Model for Stage I Adenocarcinoma of the Lung. Ann Surg Oncol 2015;22:4089-97. [Crossref] [PubMed]
- Park HJ, Park HS, Cha YJ, et al. Efficacy of adjuvant chemotherapy for completely resected stage IB non-small cell lung cancer: a retrospective study. J Thorac Dis 2018;10:2279-87. [Crossref] [PubMed]
- Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest 2002;122:1037-57. [Crossref] [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Watanabe S, Asamura H. Lymph node dissection for lung cancer: significance, strategy, and technique. J Thorac Oncol 2009;4:652-7. [Crossref] [PubMed]
- Heineman DJ, Ten Berge MG, Daniels JM, et al. The Quality of Staging Non-Small Cell Lung Cancer in the Netherlands: Data From the Dutch Lung Surgery Audit. Ann Thorac Surg 2016;102:1622-9. [Crossref] [PubMed]
- Moon Y, Kim KS, Lee KY, et al. Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1928-35. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Consolidation/Tumor Ratio on Chest Computed Tomography as Predictor of Postoperative Nodal Upstaging in Clinical T1N0 Lung Cancer. World J Surg 2018;42:2872-8. [Crossref] [PubMed]
- Heineman DJ, Ten Berge MG, Daniels JM, et al. Clinical Staging of Stage I Non-Small Cell Lung Cancer in the Netherlands-Need for Improvement in an Era With Expanding Nonsurgical Treatment Options: Data From the Dutch Lung Surgery Audit. Ann Thorac Surg 2016;102:1615-21. [Crossref] [PubMed]
- Tremblay L, Deslauriers J. What is the most practical, optimal, and cost effective method for performing follow-up after lung cancer surgery, and by whom should it be done? Thorac Surg Clin 2013;23:429-36. [Crossref] [PubMed]
- Kiankhooy A, Taylor MD, LaPar DJ, et al. Predictors of early recurrence for node-negative t1 to t2b non-small cell lung cancer. Ann Thorac Surg 2014;98:1175-83. [Crossref] [PubMed]


