Induced airway obstruction under extracorporeal membrane oxygenation during treatment of life-threatening massive hemoptysis due to severe blunt chest trauma
Introduction
It is challenging to evaluate hemorrhage and selective hemostasis in many patients with life-threatening hemoptysis in which respiratory maintenance is difficult due to airway obstruction from blood aspiration. Once life-threatening hemoptysis occurs, patients can die due to hypovolemic shock from uncontrolled bleeding or airway obstruction due to failure to remove blood clots. Thus, simultaneously improving respiratory maintenance and bleeding control increases survival of patients with life-threatening hemoptysis (1).
Extracorporeal membrane oxygenation (ECMO) has been used widely to treat acute respiratory failure in patients who are struggling to survive using a ventilator alone. Thus, ECMO can be used for respiration in patients with life-threatening massive hemoptysis in which the bleeding focus cannot be identified, and even in conditions with near-total airway obstruction. The trachea or main bronchus can be simultaneously blocked to control bleeding.
Case report
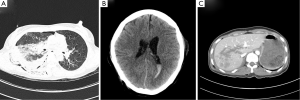
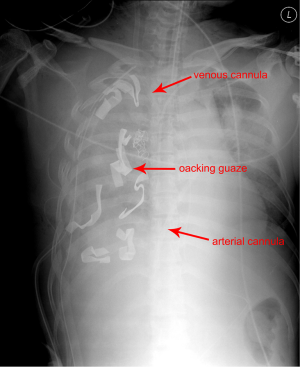
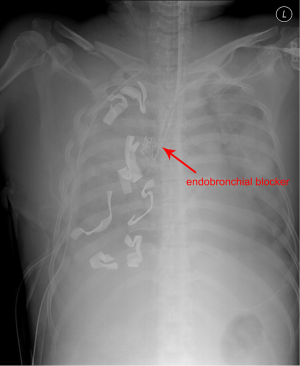
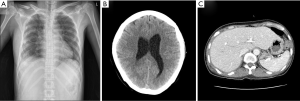
A 40-year-old female was admitted to the emergency department due to blunt trauma sustained in a traffic accident. The patient was diagnosed with intracerebral hemorrhage, severe contusion in both lungs, multiple rib fractures, right hemothorax, femur fracture, and liver laceration (Figure 1). The Glascow Coma Scale score of the patient was seven. Normal respiration was not maintained because of life-threatening massive hemoptysis; thus, venovenous (VV) ECMO (PLS System, MAQUET, Rastatt, Germany) was initiated. Emergency surgery to control left hemothorax bleeding and a wedge resection of the multiple lung laceration sites were performed under VV ECMO. Despite the surgery, the bleeding could not be controlled, and the patient was eventually transferred to the intensive care unit after gauze packing (Figure 2). However, the hemoptysis remained, so we applied endobronchial ballooning (Arndt endobronchial blocker, Cook Medical Supply, Morton, MS, USA) for hemostasis in the right main bronchus under fibrobronchoscopy. However, the tracheal bleeding did not stop, and massive hemoptysis from the left lung was also detected. Severe hemorrhage necessitated oxidized regenerated cellulose (ORC) (Surgicel, Ethicon, Somerville, NJ, USA) packing on both sides of the bronchus (Figure 3). Respiratory support was employed with ECMO but without ventilator support. The packed ORC on the left bronchus, which had less of an injury as determined by chest computed tomography, was removed on the first day of ECMO support. PaO2 and PCO2 were 70-130 and 24-32 mmHg, respectively, in ECMO blood flow of 4-5 L/min with gas flow of 3-4 L/min. The packed ORC on the right bronchus was removed on the second day. Additional hemoptysis did not occur. VV ECMO was maintained for 11 days due to acute respiratory distress syndrome. We weaned the patient from mechanical ventilation and then weaned her from ECMO. She was transfused with 50 units of red blood cells, 40 units of platelets, and 32 units of fresh frozen plasma before being removed from ECMO. We did not use anticoagulation agents until the bleeding stopped and vital signs were stable. Instead, we used a heparin-coated ECMO circuit. The femoral fracture and liver laceration were treated conservatively during ECMO. The patient received reduction surgery for the femoral fracture on day 19 after admission. The patient was discharged from the hospital on day 85. The intracranial hemorrhage and liver laceration improved spontaneously (Figure 4). The clinical progress of the patient was observed for 12 months in an outpatient clinic setting, and no further respiratory symptoms were observed. No physical or neurological deficits were detected, except for a gait disturbance due to the femoral fracture.
Discussion
Maintenance of airway patency and bleeding control should be performed simultaneously to rescue life in cases of massive hemoptysis. Then, definitive treatment such as bronchial arterial embolization (BAE) and surgery should be undertaken. However, when the volume of hemoptysis is so high that emergency hemostasis and airway maintenance are impossible due to flooding of blood into the airway, the patient dies from hypovolemia or airway obstruction without having a chance to undergo BAE or surgery. Therefore, a new life-saving rescue method needs to be developed to maintain life until definitive treatment, such as BAE and surgery, can be applied.
We thought that ECMO was ideal. ECMO is not used widely for massive hemoptysis but has been suggested in some case reports to be useful for life saving and as a bridge to treatment. Bianchini et al. (2) used ECMO as a life-saving technique in a patient with massive hemoptysis due to pulmonary artery rupture caused by a Swan-Ganz catheter and reported successful pulmonary arterial embolization. Bédard et al. (3) used ECMO as a bridge to lobectomy in patients with hypoxemia and repetitive hemoptysis due to an aortopulmonary collateral artery that occurred after a Fontan operation. Yuan et al. (4) reported the use of conservative treatment by applying ECMO in patients with massive hemoptysis due to blunt trauma.
As these reports show, sufficient blood oxygenation is possible without ventilation during maintenance with ECMO. Thus, hemostatic treatment under a non-respiratory condition is possible. We used these advantages of ECMO and actually used ECMO to execute both respiratory support and bleeding control at the same time in a patient with difficulty maintaining vital signs. Priority care in a case of respiratory failure should be focused on life rescue, and upon recovery of respiratory function, hemostasis should be performed.
Endobronchial blockade is an effective method and is preferred for emergency hemostasis. Freitag et al. (5) reported that balloon tamponade showed a 96% success rate for hemostasis in 27 patients, except one patient with short-term recurrence of hemoptysis. Valipour et al. (6) reported that ORC packing of a bleeding bronchus controlled bleeding immediately in 98% of cases, suggesting its effectiveness as a hemostasis method. However, hemostasis using endobronchial blockade requires contralateral lung protection from bleeding. This is difficult to execute in patients with massive hemoptysis and blood aspiration in both lungs. Applying ECMO can overcome these limitations.
We would have selectively packed only at the bleeding site, if it were possible to identify the site, but identifying the bleeding site was difficult because of the filled blood that made selective blocking impossible. Immediate hemostasis was needed when the exact bleeding site could not be identified. Thus, endotracheal packing using ORC was performed. In a situation where mechanical ventilation was not performed due to flooding of blood before tracheal packing, maintenance of optimal blood oxygen and carbon dioxide concentrations was possible. Thus, the patient’s vital signs remained stable even when a near total airway obstruction was induced. We rescued a life and controlled bleeding through endotracheal tamponade using ECMO in a case of life-threatening hemoptysis.
The most important factor to consider when treating massive bleeding is anticoagulation. Anticoagulation is generally necessary when using ECMO, so we thought that ECMO was not appropriate during thoracic surgery of bleeding patients despite its respiratory support advantages. However, the limitation was resolved by the introduction of the heparin-coated circuit. This circuit allows ECMO to be maintained at a lower anticoagulation level or without anticoagulation. Ried et al. reported applying only a heparin-coated circuit and avoided heparin in patients with a high risk for bleeding complications (7). We thought that strict anticoagulation was not essential. We would not use heparin in a patient who has ongoing massive bleeding because a mild coagulation defect is induced by blood loss, and hemodilution occurs due to massive crystaloid volume replacement.
In conclusion, applying ECMO could be an alternative and effective treatment in patients with respiratory failure and hypovolemic shock due to massive hemoptysis.
Acknowledgements
This study was supported by the Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital.
Disclosure: The authors declare no conflict of interest.
References
- Crocco JA, Rooney JJ, Fankushen DS, et al. Massive hemoptysis. Arch Intern Med 1968;121:495-8. [PubMed]
- Bianchini R, Melina G, Benedetto U, et al. Extracorporeal membrane oxygenation for Swan-Ganz induced intraoperative hemorrhage. Ann Thorac Surg 2007;83:2213-4. [PubMed]
- Bédard E, Lopez S, Perron J, et al. Life-threatening hemoptysis following the Fontan procedure. Can J Cardiol 2008;24:145-7. [PubMed]
- Yuan KC, Fang JF, Chen MF. Treatment of endobronchial hemorrhage after blunt chest trauma with extracorporeal membrane oxygenation (ECMO). J Trauma 2008;65:1151-4. [PubMed]
- Freitag L, Tekolf E, Stamatis G, et al. Three years experience with a new balloon catheter for the management of haemoptysis. Eur Respir J 1994;7:2033-7. [PubMed]
- Valipour A, Kreuzer A, Koller H, et al. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest 2005;127:2113-8. [PubMed]
- Ried M, Bein T, Philipp A, et al. Extracorporeal lung support in trauma patients with severe chest injury and acute lung failure: a 10-year institutional experience. Crit Care 2013;17:R110. [PubMed]


