
Clinical features of pulmonary mucormycosis in patients with different immune status
Introduction
Mucormycosis is an invasive fungal disease that is relatively rare but often fatal and rapidly progressive (1). The incidence of mucormycosis has recently been increasing (2-5). It is the second most common invasive fungal disease, ranking second only to aspergillosis. The genera that cause most cases of mucormycosis are Rhizopus, Mucor, and Lichtheimia (previously Absidia). Common predisposing factors include hematological malignancy, diabetes, transplantation, use of corticosteroids or immunosuppressants, and trauma (2,3,6). Common clinical types of mucormycosis include rhino-orbito-cerebral (27–39%), pulmonary (20–30%), dermal (19–26%), disseminated (3–15%), and gastrointestinal types (7–8.5%) (2,5,6).
Pulmonary mucormycosis (PM) is the second or third most common clinical subtype of mucormycosis (2,6). The mortality rate of PM was relatively high in early studies (56–76%) (2,7) but has recently decreased to 29% to 38% (8-10). PM continues to be a diagnostic and therapeutic challenge because its rarity makes the performance of prospective randomized controlled trials and accumulation of substantial personal experience difficult to accomplish. Two research groups reviewed the literature regarding PM at different time points (7,8); however, current studies concerning PM are mainly case reports or retrospective case series with limited numbers of patients. In addition, most studies on PM focus on severely immunocompromised patients, especially those with hematological malignancy or neutropenia (9-15). Therefore, the present study was performed to investigate the clinical manifestations, imaging features, treatment, and outcome of patients with PM with a focus on the difference in clinical manifestations between patients with different immune status.
Methods
Diagnostic criteria for PM
The present study included patients with proven or probable mucormycosis based on the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria (16). All patients had the following microbiological or histopathological evidence of mucormycosis (1): non-septate, right-angle branching filamentous fungi of variable width (6–25 µm) on direct microscopy of clinical specimens; recovery of Mucorales species by culture of clinical specimens; or confirmation of mucormycosis by histopathological examination of tissue specimens.
Study population
Patients with a diagnosis of mucormycosis were identified from medical records from January 2005 to December 2018 at Peking Union Medical College Hospital, a tertiary hospital in Beijing, China. Patients who fulfilled the criteria for proven or probable PM were included. Their case records and imaging findings were retrospectively reviewed. Observation indices included demographic information, clinical manifestations, predisposing conditions, radiologic findings, laboratory examination findings, diagnostic procedures, therapeutic interventions, and outcomes. Patients with PM were further divided into immunocompetent and immunocompromised groups for comparison.
The study was approved by the Ethics Committee of Peking Union Medical College Hospital (Reference number: S-K805). Due to the retrospective nature of the study and no identifying information relating to participants was included, written informed consent was waived.
Statistical analysis
SPSS 24.0 software (IBM Corp., Armonk, NY, USA) was used to analyze the data. Continuous variables are expressed as median and range, and qualitative variables are expressed as count and percentage. Categorical variables were compared by Fisher’s exact test, whereas continuous variables were compared by the Wilcoxon rank-sum test or Mann-Whitney U test. Two-sided P values of <0.05 were considered statistically significant.
Results
Demographic characteristics and clinical features
A total of 34 patients met the diagnostic criteria for proven or probable mucormycosis, including 20 (58.8%) with PM, 5 (14.7%) with disseminated mucormycosis (4 of whom had lung involvement), 4 (11.8%) with gastrointestinal mucormycosis, 3 (8.8%) with rhino-orbito-cerebral mucormycosis, and 2 (5.9%) with dermal mucormycosis. Among the 34 patients with mucormycosis, 24 (70.6%) had pulmonary infection involving Mucor species.
The demographic and clinical manifestations of the 24 patients with PM are shown in Table 1. The population comprised 10 female and 14 male patients with a median age of 43.5 years (range, 13–64 years). The median duration from symptom onset to the definitive diagnosis was 10 weeks (range, 2–102 weeks). Seven patients (29.2%) had an acute course (≤1 month), 9 (37.5%) had a subacute course (1–3 months), and 8 (33.3%) had a chronic course (>3 months). Common symptoms included fever in 17 patients (70.8%), cough in 17 (70.8%), sputum production in 13 (54.2%), hemoptysis in 10 (41.7%), shortness of breath in 8 (33.3%), chest pain in 4 (16.7%), and weakness in 4 (16.7%).
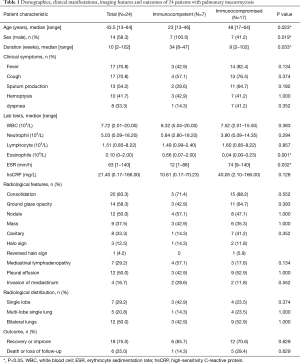
Full table
Among the 24 patients with PM, 7 (29.2%) had no obvious underlying disease; 17 (70.8%) had underlying diseases, including 7 with diabetes and 7 with hematological malignancies (4 with acute myelogenous leukemia, 1 with aplastic anemia, 1 with acute lymphoblastic leukemia, and 1 with chronic lymphocytic leukemia); and 3 had received corticosteroids and/or immunosuppressants for treatment of connective tissue disease or nephropathy.
Laboratory tests
The laboratory examination findings of the 24 patients with PM are shown in Table 1. The median leukocyte count was 7.72 (2.01–20.00) ×109/L. Four patients (16.7%) had a low leukocyte count (<4×109/L), and seven (29.2%) had a high count (>10×109/L). The median neutrophil count was 5.03 (0.09–18.20) ×109/L. The median eosinophil count was 0.10 (0–2.00) ×109/L. Four patients (16.7%) had a high eosinophil count (>0.5×109/L). Four patients (16.7%) had a high immunoglobulin E level (>2,500 KU/L). The median erythrocyte sedimentation rate was 63 [1–140] mm/h, and the median high-sensitivity C-reactive protein level was 21.40 (0.17–166.00) mg/L.
Radiological findings
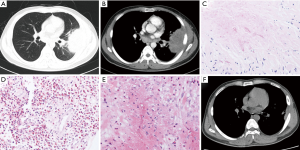
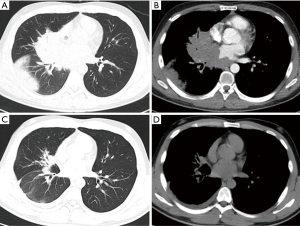
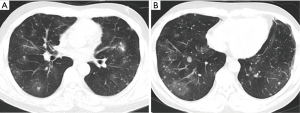
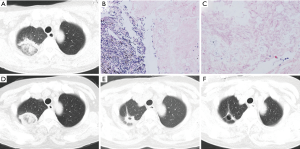
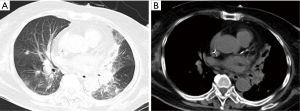
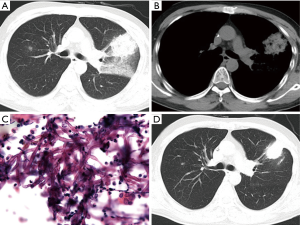
As shown in Table 1, the chest computed tomography (CT) manifestations included consolidation in 20 patients (83.3%), ground-glass opacity in 14 (58.3%), nodules in 12 (50.0%), masses in 9 (37.5%), cavities in 8 (33.3%), mediastinal lymphadenopathy in 7 (29.2%), pleural effusion in 12 (50.0%), interlobular septal thickening in 2 (8.3%), a halo sign in 3 (12.5%), and a reversed halo sign (RHS) in 1 (4.2%) (Figures 1-6). Four patients had intrapulmonary lesions invading the mediastinum, among whom two had vessel compression (Figure 2B). And one had chest wall invasion (Figure 1B). Six patients (25.0%) had a single lesion, and 18 (75.0%) had two or more lesions. The lesion distribution was as follows: 7 patients (29.2%) had a lesion in one lobe, 5 (20.8%) had lesions in multiple lobes in one lung, and 12 (50.0%) had lesions in multiple lobes in both lungs.
Diagnostic procedures
Among the 24 patients with PM, 17 (70.8%) had proven PM and 7 (29.2%) had probable PM. The diagnostic methods were histopathology in 17 (70.8%), direct microscopy of clinical specimens in 6 (25.0%), and recovery of Mucorales species from clinical specimens in 6 (25.0%) (including 3 with Rhizopus, 2 with Mucor, and 1 with Lichtheimia species). The procedures performed to obtain specimens included bronchoscopic biopsy or bronchoalveolar lavage in nine patients, percutaneous lung biopsy in six, surgical lung biopsy in four, sputum in three, pleural effusion drainage in one, and biopsy of extrapulmonary tissue in four (Figure 1E).
Pathological manifestations
The pathological characteristics of the lung tissues of 17 patients with PM were as follows (Figures 1C,D,4B,C): chronic and/or acute inflammation in 11 patients, granulomatous inflammation in 6, epithelioid granuloma in 3, necrosis in 10, eosinophilic infiltration in 3, organizing pneumonia in 1, abscesses in 1, and granulomatous angiitis in 1. Mucor species were found in the tissues of all 17 patients.
Management and outcomes
Twenty-two patients (91.7%) were treated with amphotericin B (AmB) deoxycholate. The most common adverse effect was an elevated creatinine level (n=15); other adverse effects included potassium deficiency (n=1), neutropenia (n=1), and anemia (n=1). AmB deoxycholate was stopped in four patients because of intolerance of the adverse effects. Only two patients were treated with AmB liposome. In the most recent 5 years, nine patients received posaconazole oral suspension. Only three patients underwent pulmonary lobectomy. One patient developed recurrence after surgical resection. Twelve patients improved, six were cured, three died, and three were lost to follow-up. The survival rate was 75.0%.
Comparison between immunocompetent and immunocompromised groups
The patients with PM were divided into immunocompetent and immunocompromised groups. Compared with immunocompromised patients, immunocompetent patients with PM were younger {23 [13–46] vs. 48 [17–64] years, P=0.023}, comprised a higher proportion of men (100.0% vs. 41.2%, P=0.019), had a longer disease course {34 [8–47] vs. 9 [2–102] weeks, P=0.033}, had a higher eosinophil count [0.66 (0.07–2.00) vs. 0.04 (0.00–0.23) ×109/L, P=0.001], and had a lower erythrocyte sedimentation rate {12 [1–88] vs. 74 [9–140] mm/h, P=0.032} (Table 1). Only one patient in the study (immunocompromised group) had an RHS on chest CT. No significant differences in symptoms, imaging findings, or outcomes were found (Table 1).
Discussion
This report describes our single-center experience of patients with PM with different immune status. In contrast to previous studies, our study indicates that PM could be the most common form of mucormycosis. Additionally, PM is reportedly typically acute in onset, but the infection exhibited a subacute or chronic course in two-thirds of the patients in the present study. Moreover, the clinical features differed between the immunocompetent and immunocompromised groups.
In this study, PM with or without extrapulmonary disease occurred in 70% of all patients with mucormycosis; the lung was the sole site of infection, accounting for 55.9% of patients with mucormycosis, which is much higher than previously reported (20–30%) (2,5,6). Our results suggest that pulmonary infection could be the most common form of mucormycosis, and concurrent extrapulmonary dissemination may occur in only a minority of patients (8). Further studies are needed to confirm this.
Most published cases of PM occurred in immunocompromised patients (7,8). Our results showed the most common risk factors were diabetes and hematological malignancy, consistent with previous publications (2,6-8). Mucormycosis is rarely reported in patients without predisposing conditions (17-20). In the present study, however, nearly 30% of patients with PM had no obvious underlying disease; this proportion is much higher than previously reported (12%) (7,8). In addition, the clinical features of immunocompetent patients with PM were different: most were men, younger, presented with subacute or chronic disease, and had relatively less severe systemic inflammation compared with immunocompromised patients. The reason for the development of PM in immunocompetent patients is unclear but may be associated with individual susceptibility or exposure to a large amount of Mucor microorganisms with high virulence (18). Thus, mucormycosis can infect a variety of patient groups, including severely immunodeficient patients, such as those with hematological malignancies and those who have undergone transplantations; patients using immunosuppressants; patients who are only mildly immunocompromised, such as those with diabetes; and even immunocompetent patients. Patients with different immune status may have different clinical phenotypes.
The clinical manifestations of PM are nonspecific. In the present study, common symptoms included fever, cough, expectoration, and hemoptysis, which is consistent with a previous study (8). Although most patients with PM present with fulminant and rapidly progressive disease, our study revealed that a substantial number of patients can manifest chronic symptoms, as occasionally reported in the literature (21). In addition, our study showed that invasion of contiguous chest structures (chest wall, pericardium, mediastinum) is an unusual complication of PM. PM can also reportedly involve the recurrent laryngeal nerve, leading to vocal cord paralysis (22,23); compress the sympathetic chain, resulting in Horner’s syndrome (24); or penetrate the bronchial wall, leading to chronic mediastinitis (25). PM can also invade the chest wall, leading to subcutaneous emphysema, or extend from the lung to abdominal organs (transdiaphragmatic mucormycosis) (26,27).
The radiologic features of PM are also nonspecific and exhibit a wide spectrum of types. In the present study, the common chest CT findings were nodules, masses, or consolidation shadows with or without cavities; these findings are consistent with previous studies (7,8,13,15). The RHS is considered a characteristic imaging finding of PM, and its incidence may reach 54% to 94% (9,12,14,28). In contrast to previous studies, the incidence of the RHS in our study group was very low (4.2%). One possible explanation could be differences in the hosts’ immune status. The prevalence of the RHS among patients with PM with hematological malignancies or neutropenia is very high (9,12). While few patients in the present study had these diseases, a substantial number of patients were immunocompetent or mildly immunocompromised (i.e., diabetes). Another explanation might be that the CT scans were performed at different stages of the disease. The RHS in most patients with neutropenia occurs within the first week (9,28). In our study, however, a portion of patients presented with a chronic course, and CT was not performed in the early stage. In severely immunocompromised patients, especially those with hematological malignancies and neutropenia, the RHS is highly suggestive of early infection by Mucorales species and can be useful for preemptive initiation of antifungal therapy (9,12,29). However, for mildly immunocompromised patients (such as those with diabetes) or immunocompetent patients, the diagnostic value of the RHS for PM remains uncertain because this nonspecific finding is commonly seen in a wide range of infectious and noninfectious pulmonary diseases (29). In the case of pulmonary tuberculosis infection, it has been noted that severe immune suppression may limit the development of a radiographically observable tuberculosis response to infection (30,31). However, this pattern was not demonstrated in our cohort of PM.
In this study, a few patients with PM had an elevated eosinophil level in the peripheral blood or infiltration of eosinophils in lung tissue. The most common etiology of an elevated eosinophil level caused by infectious disease is parasite infestation, but some fungal infections such as coccidioides also cause hypereosinophilia (32-34). Paracoccidioidomycosis, disseminated histoplasmosis, and cryptococcosis can also occasionally cause a high blood eosinophil level. Mucormycosis with increased eosinophils has occasionally been reported (35,36). The role of eosinophils in the pathogenesis of mucormycosis requires further investigation.
The diagnosis of PM is still challenging because of the nonspecific clinical manifestations and imaging features and lack of effective serum markers. Thus, the key to a definitive diagnosis is microbiological or histopathologic evidence (1). In the present study, histopathological examination of tissue specimens had the highest diagnostic yield, followed by direct microscopy or culture of clinical specimens. Therefore, active tissue biopsy is very important for early diagnosis. The biopsy specimens should be delivered for simultaneous pathological examination, direct microscopic examination, and culture (1). Bronchoalveolar lavage fluid, sputum, and pleural effusion specimens are more accessible than biopsy specimens and may also be helpful in the diagnosis. In the present study, about 60% of patients were diagnosed via bronchoscopy or percutaneous puncture, and only a few patients needed a more invasive procedure such as surgical lung biopsy. Other methods that may be helpful for early diagnosis of PM need to be explored, such as the use of molecular methods on both fresh clinical material and paraffin slides, Mucorales-reactive lymphocyte assay on peripheral blood, and quantitative polymerase chain reaction assays for circulating Mucorales detection (1,37,38).
Treatment for mucormycosis includes removal of risk factors, implementation of anti-Mucor therapy, and surgical resection in selective patients. The European Society for Clinical Microbiology and Infectious Diseases and the European Confederation of Medical Mycology recommended liposomal AmB as a first-line treatment and posaconazole as a first-line or salvage treatment (1). However, a huge gap exists between clinical practice and these guidelines because antifungal drugs are either very expensive or unavailable. Most patients in the present study were treated with AmB deoxycholate, which is not recommended in the guideline because of its substantial toxicity. Posaconazole became available in the past several years and has thus provided an alternative choice. Nonetheless, the treatment response rate in the present study was more than two-thirds, and the survival rate was slightly higher than in previous studies (2,7-9). A possible explanation is that most of the patients were immunocompetent or mildly immunocompromised. The combination of surgery with medical treatment in patients with mucormycosis is strongly recommended because surgery seems to significantly improve survival (1,2,7,9,39). However, most evidence concerning surgical treatment has been obtained from case reports or small retrospective case series (7,9,39,40). In the present study, most patients responded well to sole antifungal therapy. About three-fourths of patients had multiple lung lesions and were therefore unsuitable for surgical treatment. One patient developed recurrence after surgical resection (18). Therefore, with the emergence of more novel antifungal agents, further studies are needed to evaluate the value of surgical treatment and clarify which patients will benefit most from surgical treatment.
Our study had several limitations, mainly its small size and retrospective nature. Furthermore, because our patients had various host factors, one should be careful when applying these findings to other patients with different risk factors. Finally, because nearly 30% of the cases were probable infections, firm conclusions are difficult to make.
In conclusion, PM can occur in heterogeneous patients with different immune status, and the clinical phenotype differs between immunocompetent and immunocompromised patients. Because of the lack of specific clinic and imaging manifestations, aggressive performance of invasive procedures to obtain histopathological and microbial evidence is crucial for a definitive diagnosis.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of Peking Union Medical College Hospital (Reference number: S-K805).
References
- Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect 2014;20 Suppl 3:5-26. [Crossref] [PubMed]
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634-53. [Crossref] [PubMed]
- Petrikkos G, Skiada A, Drogari-Apiranthitou M. Epidemiology of mucormycosis in Europe. Clin Microbiol Infect 2014;20 Suppl 6:67-73. [Crossref] [PubMed]
- Bitar D, Van Cauteren D, Lanternier F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997-2006. Emerg Infect Dis 2009;15:1395-401. [Crossref] [PubMed]
- Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect 2011;17:1859-67. [Crossref] [PubMed]
- Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 2019;25:26-34. [Crossref] [PubMed]
- Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med 1999;159:1301-9. [Crossref] [PubMed]
- Feng J, Sun X. Characteristics of pulmonary mucormycosis and predictive risk factors for the outcome. Infection 2018;46:503-12. [Crossref] [PubMed]
- Legouge C, Caillot D, Chretien ML, et al. The reversed halo sign: pathognomonic pattern of pulmonary mucormycosis in leukemic patients with neutropenia? Clin Infect Dis 2014;58:672-8. [Crossref] [PubMed]
- Lin E, Moua T, Limper AH. Pulmonary mucormycosis: clinical features and outcomes. Infection 2017;45:443-8. [Crossref] [PubMed]
- Chamilos G, Marom EM, Lewis RE, et al. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis 2005;41:60-6. [Crossref] [PubMed]
- Bourcier J, Heudes PM, Morio F, et al. Prevalence of the reversed halo sign in neutropenic patients compared with non-neutropenic patients: Data from a single-centre study involving 27 patients with pulmonary mucormycosis (2003-2016). Mycoses 2017;60:526-33. [Crossref] [PubMed]
- Nam BD, Kim TJ, Lee KS, et al. Pulmonary mucormycosis: serial morphologic changes on computed tomography correlate with clinical and pathologic findings. Eur Radiol 2018;28:788-95. [Crossref] [PubMed]
- Jung J, Kim MY, Lee HJ, et al. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin Microbiol Infect 2015;21:684.e11-8. [Crossref] [PubMed]
- Jin S, Zhang B, Zhang L, et al. Lung nodules assessment in ultra-low-dose CT with iterative reconstruction compared to conventional dose CT. Quant Imaging Med Surg 2018;8:480-90. [Crossref] [PubMed]
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. [Crossref] [PubMed]
- Lee JS, Kim HC, Park SW, et al. A case of isolated pulmonary mucormycosis in an immunocompetent host. Tuberc Respir Dis (Seoul) 2013;74:269-73. [Crossref] [PubMed]
- Zhang L, Tian X, Wang P, et al. Recurrent pulmonary mucormycosis after lobectomy in a non-smoking patient without predisposing risk factors. Braz J Infect Dis 2012;16:590-3. [Crossref] [PubMed]
- Acharya S, Shukla S, Noman O, et al. Isolated pulmonary mucormycosis presenting as cavitary lesion in an immunocompetent adult: A rare case report. Int J Appl Basic Med Res 2016;6:73-4. [Crossref] [PubMed]
- Sharma A, Gupta V, Singh RS, et al. Angioinvasive pulmonary mucormycosis presenting as multiple bilateral pulmonary nodules in a patient without obvious predisposing factors. Singapore Med J 2008;49:e269-71. [PubMed]
- Agarwal R, Kumar V, Gupta D. Pulmonary mucormycosis: two of a kind. Eur J Intern Med 2006;17:63-5. [Crossref] [PubMed]
- Gayathri Devi HJ, Mohan Rao KN, Prathima KM, et al. Pulmonary mucormycosis presenting with vocal cord paralysis. Respir Med Case Rep 2013;9:15-7. [Crossref] [PubMed]
- Mahadevaiah AH, Rajagopalan N, Patil M, et al. Coinfection of pulmonary mucormycosis and aspergillosis presenting as bilateral vocal cord palsy. BMJ Case Rep 2013. [Crossref] [PubMed]
- Kotoulas C, Psathakis K, Tsintiris K, et al. Pulmonary mucormycosis presenting as Horner's syndrome. Asian Cardiovasc Thorac Ann 2006;14:86-7. [Crossref] [PubMed]
- Liu HC, Jan MS, Lin YC, et al. A rare pulmonary zygomycosis manifested as chronic mediastinitis and bronchial perforation. Eur Respir J 2011;38:734-5. [Crossref] [PubMed]
- Koshy CG, Shah S, Mammen T. Subcutaneous emphysema of the chest: could it be pulmonary mucormycosis? Thorax 2010;65:280. [Crossref] [PubMed]
- Koehler P, Reimer R, Wahba R, et al. Transdiaphragmatic Mucormycosis. Clin Infect Dis 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Wahba H, Truong MT, Lei X, et al. Reversed halo sign in invasive pulmonary fungal infections. Clin Infect Dis 2008;46:1733-7. [Crossref] [PubMed]
- Georgiadou SP, Sipsas NV, Marom EM, et al. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis 2011;52:1144-55. [Crossref] [PubMed]
- Fishman JE, Sais GJ, Schwartz DS, et al. Radiographic findings and patterns in multidrug-resistant tuberculosis. J Thorac Imaging 1998;13:65-71. [Crossref] [PubMed]
- Wáng YXJ, Chung MJ, Skrahin A, et al. Radiological signs associated with pulmonary multi-drug resistant tuberculosis: an analysis of published evidences. Quant Imaging Med Surg 2018;8:161-73. [Crossref] [PubMed]
- Harley WB, Blaser MJ. Disseminated coccidioidomycosis associated with extreme eosinophilia. Clin Infect Dis 1994;18:627-9. [Crossref] [PubMed]
- Malo J, Luraschi-Monjagatta C, Wolk DM, et al. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc 2014;11:243-53. [Crossref] [PubMed]
- Simons CM, Stratton CW, Kim AS. Peripheral blood eosinophilia as a clue to the diagnosis of an occult Coccidioides infection. Hum Pathol 2011;42:449-53. [Crossref] [PubMed]
- Sato M, Gemma H, Sano T, et al. Pulmonary mucormycosis caused by Cunninghamella bertholletiae in a non-immunocompromised woman. Nihon Kokyuki Gakkai Zasshi 2001;39:758-62. [PubMed]
- Hirano T, Yamada M, Sato K, et al. Invasive pulmonary mucormycosis: rare presentation with pulmonary eosinophilia. BMC Pulm Med 2017;17:76. [Crossref] [PubMed]
- Steinbach A, Cornely OA, Wisplinghoff H, et al. Mould-reactive T cells for the diagnosis of invasive mould infection-A prospective study. Mycoses 2019;62:562-9. [Crossref] [PubMed]
- Millon L, Larosa F, Lepiller Q, et al. Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin Infect Dis 2013;56:e95-101. [Crossref] [PubMed]
- Tedder M, Spratt JA, Anstadt MP, et al. Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg 1994;57:1044-50. [Crossref] [PubMed]
- Vercillo MS, Liptay MJ, Seder CW. Early pneumonectomy for pulmonary mucormycosis. Ann Thorac Surg 2015;99:e67-8. [Crossref] [PubMed]


