Cystic fibrosis patients at risk for disease progression marked by decline in FEV1% predicted: development of the cystic fibrosis risk of disease progression score
Introduction
In the United States cystic fibrosis (CF) is second only to sickle cell anemia as the most common life shortening, recessively inherited disorder typically diagnosed in infancy or early childhood. Approximately 30,000 individuals in the United States have CF with an estimated 1,000 new cases diagnosed each year (1,2). The disease may remain asymptomatic in early childhood, depending on severity. Progression to symptomatic disease state often manifests with mucoviscidosis blocking the airways leading to inflammation with secondary infections and potential for lung damage. Pulmonary exacerbation resulting from disease progression is a major contributor to morbidity and mortality (3-5). Characteristics defining a pulmonary exacerbation include decline in lung function (3,4,6). Limited data describe variables contributory to a sharp decline in FEV1% predicted over a relatively short interval of time; significant lung function decline often results in lung transplantation candidacy or premature death. Determining the phenotypic markers of CF patients likely to experience a significant drop in FEV1% predicted (10 or more percentage points from baseline) will help target efforts for prevention of this deleterious decline. Areas for address may include exposure reduction strategies, therapeutic interventions, and promotion of healthy lifestyle management.
Current models of CF marker analytics have not provided clinically useful results. The first scoring method for CF, the Shwachman-Kulczycki (SK) score was published in 1958 (7) and is based on chest radiological and clinical evaluation. While subsequent scores have been developed, many are alterations of the original SK score method, such as those published by Stollar et al. and Brasfield et al. (8,9). Nkam et al. developed a multivariable logistic regression model that predicted death or lung transplant using data gathered from the French CF patient registry (10). These models are quite complex, and unlike our proposed scoring method, none of them can predict decline in FEV1%. If we could define: (I) when patients will decline; (II) characterize SA’s role in this decline, we can plan the bridge to transplant more specifically.
CF management guidelines recommend routine clinical monitoring of CF patients (11). This includes categorization of severity based on FEV1% predicted at least four times per year, comprehensive lung function assessment at least every 12 months, and cultures of respiratory tract secretions four times per year. The objective of this study was to determine whether various routinely collected and analyzed demographic and clinical characteristics are predictive of accelerated disease progression across a two-year follow-up period. The aim of this analysis was to (I) identify those factors that contribute to disease progression; (II) be able to predict which patients are at a high risk of decline of FEV1% predicted ≥10 percentage points referred to as the cystic fibrosis risk of disease progression (CF RD-Pro) score.
Methods
Study population
This study evaluated potential factors that can account for accelerated decline in FEV1% predicted in 81 CF patients treated at the Cystic Fibrosis Center at MCWHLB. These patients were selected from the CF-Port database during the 5-year period January 1, 2007-December 13, 2011 based on the following inclusion criteria: (I) FEV1%-predicted across health encounters in baseline three month interval ≥60%; (II) patient returned for care in the subsequent three to four months intervals and FEV1%-predicted determined and documented in database. All patients treated at the single center who were identified with CF port data during respective time period and met study criteria comprised the study cohort. Baseline encompassed a three-month period from point of first documented health encounter in database during study period. Patients were monitored across 409 three-month intervals (865 health encounters) until drop in FEV1% predicted ≥10% or patient censored (maximum 3-year follow-up without event (significant drop in FEV1%) or lost to follow-up before event). The study was approved by the MemorialCare Institutional Review Board, Long Beach.
Measures and data collection
The best spirometric measure of at least three attempts was recorded in CF-Port database at each documented health encounter. Normal FEV1 predictive value per Knudson/Intermountain Thoracic Society (12) was adjusted for ethnicity and reported in database as FEV1% predicted. Minimum FEV1% predicted across health encounters during each interval was evaluated and compared to the prior interval. A drop in FEV1% predicted of ≥10 percentage points from one interval to the next marked significant disease progression (referred to as ‘event’). Patients were monitored across all follow-up intervals until event occurred or patient censored. Additional CF-Port data accessed for analysis include age at diagnosis, sex, BMI (height and weight), Tobramycin by inhalation (TOBI) medication use, FEV1% predicted, FVC% predicted at baseline, and culture results (Staphylococcus aureus and Pseudomonoas aeruginosa). A chart review was conducted to abstract additional clinical information including whether bronchoscopy performed (Y/N), bronchoscopy impact score (mild, moderate, severe), sinus surgery performed (Y/N), and chest X-ray performed (Y/N) and results: scarring [yes~location (lower, upper, both) or no], bronchiectasis (Y/N), and/or cystic formation (Y/N). Databases were linked to capture patient demographics and experience during concurrent three-month intervals.
Statistical methods
The distribution of patient characteristics in our 81 cystic fibrosis patients was described in terms of valid percent with defined trait or mean [standard deviation (SD)] for continuous factors. Cox regression analyses were conducted with intent to treat approach to assess the influence of patient characteristics on the cumulative probability that a patient would experience a significant decline in FEV1% predicted (≥10 percentage points) with each subsequent three-month interval. The TIES=EXACT approach was specified in the model for PHREG procedure in SAS (SAS Institute, Inc, Cary, NC, USA) to account for the possibility of multiple patients experiencing event (marked decline in FEV1% predicted) during a particular interval. The time varying covariate approach was applied by use of long data format and method = CH specified in procedure statement in SAS. The hazard rate of event for each potential influential factor level compared to the reference category was estimated and 95% CIs reported. Factors showing differential hazard of event across categories informed item inclusion and quantification of CF RD-Pro score.
CF RD-Pro score was evaluated as tool to identify high-risk patients for disease progression (with drop in FEV1% predicted ≥10%) in subset of 50 patients monitored for two years by study endpoint among our center cohort. Scale items were assessed during year one and total score tested using ROC analysis to determine high-risk threshold of event occurring during year two. Identified threshold for high versus low-moderate risk was compared against occurrence of year two event and validation measures reported: sensitivity, specificity, predictive value positive, negative predictive value, and percent correct classification. Logistic regression analysis determined likelihood of event occurrence during year two based on patients CF RD-Pro score (≥2 vs. <2). Cox regression analyses were performed using SAS v9.0 and all remaining analysis conducted using SPSS v18.0.
Results
Patient characteristics and factors influencing drop in FEV1% predicted (≥10% points)
Table 1 describes the patient population with variables likely to accelerate disease progression. In our predominately pediatric patient population (81.5%), 49.4% were male and 65.4% were 6–12 years of age. Mean BMI was 19.9 (SD =4.6) and 37.0% had BMI values >20 (24.4% of female and 40.0% of male children). Lung function at baseline, on average, was suggestive of mild disease severity (mean FEV1% predicted=86.8%, SD =16.0); 69.1% of patients had FEV1% predicted value ≥80%. In terms of FVC%-predicted at baseline the average value was 96.3% (SD =15.0). During the period of time in which a patient was considered to be at risk, 75.3% experienced a Staphylococcus infection (38.3% two or more). In the 61 patients positive for Staphylococcus infection, 46 (75.4%) were positive to methicillin-susceptible Staphylococcus aureus (MSSA) only, 8 (13.1%) were positive to methicillin-resistant Staphylococcus aureus (MRSA) only, and 7 (11.5%) had one or more positive tests for both MSSA & MRSA. Approximately 39.5% of patients demonstrated positive Pseudomonas aeruginosa infection, not classified for mucoid or non-mucoid type (12.3% two or more). TOBI use was reported by 54.3% at baseline and by 63.0% through interval preceding last follow-up or event. Chart review revealed 13.6% had a bronchoscopy performed during observation period, 17.3% had sinus surgery, and 42.0% had a chest roentgenogram (X-ray). In 18 patients with scarring noted on examination of chest X-ray, location was predominately in the upper regions (n=14, 77.8%). Staphylococcus infection(s) and >20 BMI in female pediatric patients increased hazard of significant drop in FEV1% predicted (≥10%) in any interval (P<0.05).
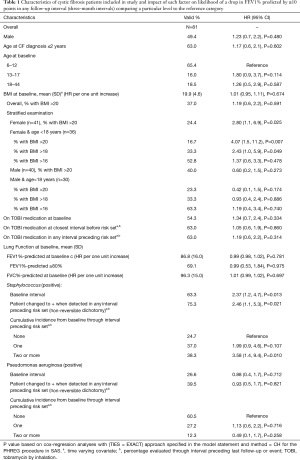
Full table
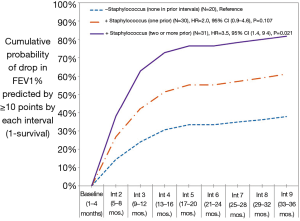
The cumulative probability of significant loss in pulmonary function was 43% by end of year one (interval 3), 58% by end of year two (interval 6), and 65% by end of year three (interval 9). Accelerated loss of lung function was significantly influenced by prior number of Staphylococcus aureus infections (none, one, two or more) (Figure 1). Patients with two or more prior Staphylococcus aureus infections were 3.5 times more likely to experience a drop in FEV1% predicted (≥10%) in any interval than patients with none reported in prior intervals (95% CI, 1.9–9.4, P=0.021). By the end of year three, the cumulative probability of a ≥10 percentage point drop in FEV1% predicted was 37.8% in patients with no prior Staphylococcus infections, 61.1% in patients with one prior infection, and 81.7% in patients with two or more prior infections. In female patients, those whose BMI >20 were 2.8 times more likely than those whose BMI ≤20 to experience a significant drop in FEV1% predicted (HR 2.80; 95% CI, 1.1–6.9; P=0.025). In male patients, differential risk by BMI was not observed at threshold >20 nor when analyzed at higher threshold values extending up to >25. Hazard of event did not significantly differ by whether bronchoscopy performed (limited data to assess impact score on outcome), sinus surgery, nor by indication of scarring on review of chest X-ray (data not shown); however, these results should be viewed with caution as fewer than half of patients had a chest X-ray during concurrent review period and less than 20% had bronchoscopy and/or sinus surgery performed.
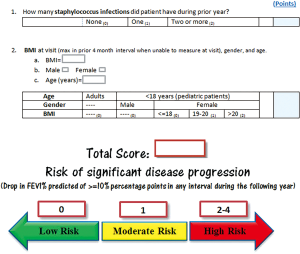
CF RD-Pro score
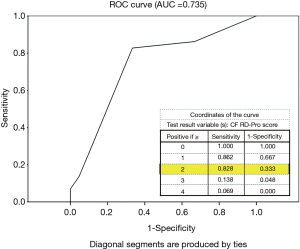
Item selection to quantify CF RD-Pro marked by significant drop in FEV1% predicted (≥10%) was informed by cox-regression results. In 50 patients monitored for 2 years by study end-point, a total of 29 (58%) experienced accelerated disease progression in year two. CF RD-Pro scores were calculated for each patient based on number of Staphylococcus infections during year one and their BMI at end of year one with consideration to age (pediatric or adult) and sex (Figure 2). ROC analysis determined classification of high versus low-moderate risk of event occurrence during year two based on RD-Pro score provided an area under the curve (AUC) =0.735 (Figure 3). Scores ≥2 applied as threshold for high-risk revealed relatively good validity estimates: sensitivity =82.8%, specificity =66.7%, PVP =77.4%, PVN =73.7%, and correct classification =76%. Patients with CF RD-Pro scores suggestive of high (≥2 points) vs. low-moderate (<2 points) risk were nearly 10 times more likely to experience significant disease progression (OR 9.6; 95% CI, 2.6–36.1; P=0.001). High risk was detected in patients with two or more prior Staphylococcus infections; specific to female pediatric patients, additional high-risk criteria include BMI>20 or (one or more prior Staphylococcus infections and BMI >18).
Discussion
FEV1% predicted at baseline was suggestive of mild pulmonary impairment in the majority of our patients (baseline FEV1% predicted ≥80% in 69% of patients). However, the cumulative probability of significant loss in pulmonary function was marked by a drop in FEV1% predicted of ≥10 percentage points in each subsequent 3 months interval was 43% by end of year one, 58% by end of year two, and 65% by end of year three. Thus, our patient population represents a typical CF patient cohort. Early intervention in CF relies on early prediction of lung function decline. Such intervention also includes addressing the healthy subset, a subset that tends to be difficult to predict decline of their FEV1. Hence, our focus is on patients with up to moderate disease. The instability of moderately severe and severe patients would limit the accuracy of our statistical focus. Upon further validation across larger cohorts, the application of this score to the severe population would be appropriate. Remarkably, monitoring FEV1% predicted across time enabled identification of significant disease progression in patients who may have otherwise been considered to have mild disease based on an annual point in time assessment. Accelerated loss of lung function was significantly influenced by prior number of Staphylococcus infections (none, one, two or more) and elevated BMI in female patients. Patients were detected at high risk for significant decline in lung function when they had two or more prior Staphylococcus infections. In female pediatric patients, high risk was also detected when BMI >20 or when patient had one prior Staphylococcus infection and BMI >18. Translation of these findings into CF RD-Pro score provided a valid tool to identify high-risk patients.
Nearly 75% of our CF patient population had a SA infection by study end point. In a cohort of 413 adults and children identified in two large CF centers affiliated with Paris Descartes University presence of SA infection was comparable at 72% (13). Investigators in this study found SA prevalence rates were similar in adults and children. Similar to our findings, MSSA was also more prevalent than MRSA (13). MRSA was present in 24% of patients in their cohort (24.6% of our patients had an MRSA infection by study end point: 13.1% had an MRSA infection without incidence of MSSA infection and 11.5% had both MRSA and MSSA infections). Interestingly, they found SA corresponded to lower average FEV1% predicted and particularly with a MRSA strain when Pseudomonas aeruginosa (PA) was also present. Presence of SA also corresponded to yearly decline in average FEV1% predicted (MSSA alone or MRSA combined with PA) (13). Similarly, we found that prior SA infection(s) corresponded to increased hazard of a marked drop in FEV1% predicted in any subsequent three-month interval. Although we had limited data to evaluate the effect of strains (MSSA vs. MRSA) on accelerated decline, recent studies suggest that presence of MRSA may correspond to worse outcomes than observed with presence of MSSA (14,15) and feasibly MRSA could correspond to even greater risk of accelerated decline in FEV1% predicted than observed for MSSA. Sawicki et al. showed correspondence of MRSA to lower average FEV1% predicted but detection was not associated with changing rate of FEV1% predicted decline in a population of approximately 5000 children evaluated during years 2001–2003 (16). In a large cohort of 17,357 patients identified in the Cystic Fibrosis Foundation patient registry, Dasenbrook and colleagues showed average decline in FEV1% predicted was 43% more rapid in patients with persistent MRSA than those MRSA negative (P<0.05) (14). Ren et al. [2007] found in a large observational study of 20,451 CF patients, 6–17 years of age residing in North American during 2001, children with MRSA had significantly lower average FEV1% compared to patients with MSSA (15). Hubert et al. showed significant yearly decline in patients with MSSA alone and near significance in presence of MRSA alone compared to patients with No SA no PA (13). A shift in treatment complexity may have been a contributing factor to more notable differentials in our SA positive compared to SA negative groups. Only TOBI medication use was examined in our evaluation for potential confounding effects on relationship between SA and accelerated decline in FEV1% predicted and found to be non-significant. With rising frequency of MRSA infection (14), further investigation of outcome differentials between patients positive to each strain is merited. Nevertheless, corroborated by prior studies, our findings clearly suggest prevention of SA of either type (MSSA or MRSA) will contribute to better patient prognosis (17).
An association between Pseudomonoas aeruginosa and impaired lung function has been reported in several prior studies albeit not in a single year time interval (14,18,19). Our study did not find PA infection corresponded to an accelerated decline in FEV1% predicted nor influence the direct relationship observed with number of prior SA infections. This could in part be explained by high reported use of TOBI (76%) in intervals when concurrent PA infection noted which has previously been shown to reduce decline in FEV1% predicted (20).
To our knowledge no studies to date show correspondence of increased BMI (>20) to accelerated lung function decline in female children as found in our study. This same trend was not seen in male patients. In a related study of 109 children 7–17 years of age treated at HCS and Montreal Children’s Hospital (MCH) CF, girls in two lowest activity quartiles had more rapid rate of decline in FEV1% predicted than the two highest activity quartiles, which was not observed in boys (21). Cystic Fibrosis Foundation Patient Registry 2015 Annual Data Report suggests CF children to maintain a BMI around the 50th percentile. Kerem et al. found that low BMI significantly increased the odds of severe lung disease (FEV1% predicted <40%) (22). Stallings et al. (23) referenced studies by Wang et al. (24) and Hankinson et al. (25) that showed higher BMI percentile in children 6–12 and 13–20 years of age, respectively, corresponded to higher average FEV1% predicted. Directionally similar results were found by Forte et al. who reported BMI >50th percentile preserved pulmonary function as defined by FEV1% predicted >80% (26). Panagopoulou et al. suggest intensive monitoring for both malnutrition and overweight/obesity in CF patients which prior literature and our current study substantiate (27).
Limitations
This is an observational study based on registry data. Balancing nutritional sufficiency and lung function may indeed be influenced by sex compounded by other disease modifiers. Our small study population, in this respect, will require further research in larger cohorts. Confounding effects of factors not measured or not standardized in the registry which further define treatment regimen may have impacted our patient population results. It is anticipated on the next validation study of this model that modulator therapy will be organized for appropriate analysis. All patients were identified from the same clinic, the Cystic Fibrosis Center at Long Beach Memorial, CA, during the study period increasing likelihood of standard exposure to education and access to programs related to nutrition and physical activity while also minimizing bias due to potential site of care clustering effects. A total of 220 patients were identified in CF port as treated at the Cystic Fibrosis Center from January 1, 2007 through August 13, 2011. Patients were excluded due to detection of severe lung disease (FEV1% predicted <60%) in baseline interval (exclusion criteria). In 120 patients with minimum FEV1% predicted in baseline interval ≥60%, 22.5% did not have follow-up care documented in CF Port data in the next three-month interval. An additional 12.9% were excluded due to non-documentation of FEV1% predicted in the three-month period following the baseline interval. In the final 81 patients included in evaluation average baseline FEV1% predicted was similar to the 39 patients who met eligibility criteria but were excluded due to lack of follow-up in specified time period or were missing FEV1% predicted in the next interval (86.8% vs. 83.8%, P=0.332).
Conclusions
Novel scoring systems are needed in CF particularly serving to identify patients at risk of rapid lung function decline increasing the likelihood of lung transplantation. Utilization of the CF RD-Pro score requires study in larger CF population to further validate its efficacy in predicting a one-year decline in lung function. CF RD-Pro score is simple to calculate and provides a relatively good predictive value detecting patients at high risk for accelerated decline in lung function (drop in FEV1% predicted ≥10 percentage points). Patients with CF RD-Pro scores suggestive of high (≥2 points) vs. low-moderate (<2 points) risk were nearly 10 times more likely to experience significant disease progression (OR 9.6, 95% CI, 2.6–36.1, P=0.001). Identification of patients at high risk for significant decline in lung function will enable address of potential mitigating treatment, environmental exposures, and behavioral variants to improve outcomes in these high-risk patients. Management of Staphylococcus aureus infection via preventive measures and targeted therapeutic intervention are key directives of high CF RD-Pro scores.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the MemorialCare Institutional Review Board, Long Beach.
References
- Agrawal A, Agarwal A, Mehta D, et al. Nationwide trends of hospitalizations for cystic fibrosis in the United States from 2003 to 2013. Intractable Rare Dis Res 2017;6:191-8. [Crossref] [PubMed]
- Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev 2010;23:299-323. [Crossref] [PubMed]
- Sanders DB, Bittner RC, Rosenfeld M, et al. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010;182:627-32. [Crossref] [PubMed]
- Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax 2007;62:360-7. [Crossref] [PubMed]
- Kerem E, Reisman J, Corey M, et al. Prediction of mortality in patients with cystic fibrosis. N Engl J Med 1992;326:1187-91. [Crossref] [PubMed]
- Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 1994;331:637-42. [Crossref] [PubMed]
- Shwachman H, Kulczycki LL. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J Dis Child 1958;96:6-15. [Crossref] [PubMed]
- Stollar F, Adde FV, Cunha MT, et al. Shwachman-Kulczycki score still useful to monitor cystic fibrosis severity. Clinics (Sao Paulo) 2011;66:979-83. [Crossref] [PubMed]
- Brasfield D, Hicks G, Soong S, et al. The chest roentgenogram in cystic fibrosis: a new scoring system. Pediatrics 1979;63:24-9. [PubMed]
- Nkam L, Lambert J, Latouche A, et al. A 3-year prognostic score for adults with cystic fibrosis. J Cyst Fibros 2017;16:702-8. [Crossref] [PubMed]
- Saiman L, Siegel JD. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infect Control Hosp Epidemiol 2014;35 Suppl 1:S1-67. [Crossref] [PubMed]
- Knudson RJ, Lebowitz MD, Holberg CJ, et al. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 1983;127:725-34. [PubMed]
- Hubert D, Reglier-Poupet H, Sermet-Gaudelus I, et al. Association between Staphylococcus aureus alone or combined with Pseudomonas aeruginosa and the clinical condition of patients with cystic fibrosis. J Cyst Fibros 2013;12:497-503. [Crossref] [PubMed]
- Dasenbrook EC, Merlo CA, Diener-West M, et al. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med 2008;178:814-21. [Crossref] [PubMed]
- Ren CL, Morgan WJ, Konstan MW, et al. Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol 2007;42:513-8. [Crossref] [PubMed]
- Sawicki GS, Rasouliyan L, Pasta DJ, et al. The impact of incident methicillin resistant Staphylococcus aureus detection on pulmonary function in cystic fibrosis. Pediatr Pulmonol 2008;43:1117-23. [Crossref] [PubMed]
- Waters V, Atenafu EG, Lu A, et al. Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J Cyst Fibros 2013;12:482-6. [Crossref] [PubMed]
- Que C, Cullinan P, Geddes D. Improving rate of decline of FEV1 in young adults with cystic fibrosis. Thorax 2006;61:155-7. [Crossref] [PubMed]
- Navarro J, Rainisio M, Harms HK, et al. Factors associated with poor pulmonary function: cross-sectional analysis of data from the ERCF. European Epidemiologic Registry of Cystic Fibrosis. Eur Respir J 2001;18:298-305. [Crossref] [PubMed]
- VanDyke RD, McPhail GL, Huang B, et al. Inhaled tobramycin effectively reduces FEV1 decline in cystic fibrosis. An instrumental variables analysis. Ann Am Thorac Soc 2013;10:205-12. [Crossref] [PubMed]
- Schneiderman-Walker J, Wilkes DL, Strug L, et al. Sex differences in habitual physical activity and lung function decline in children with cystic fibrosis. J Pediatr 2005;147:321-6. [Crossref] [PubMed]
- Kerem E, Viviani L, Zolin A, et al. Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS patient registry. Eur Respir J 2014;43:125-33. [Crossref] [PubMed]
- Stallings VA, Stark LJ, Robinson KA, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc 2008;108:832-9. [Crossref] [PubMed]
- Wang X, Dockery DW, Wypij D, et al. Pulmonary function growth velocity in children 6 to 18 years of age. Am Rev Respir Dis 1993;148:1502-8. [Crossref] [PubMed]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179-87. [Crossref] [PubMed]
- Forte GC, Pereira JS, Drehmer M, et al. Anthropometric and dietary intake indicators as predictors of pulmonary function in cystic fibrosis patients. J Bras Pneumol 2012;38:470-6. [Crossref] [PubMed]
- Panagopoulou P, Fotoulaki M, Nikolaou A, et al. Prevalence of malnutrition and obesity among cystic fibrosis patients. Pediatr Int 2014;56:89-94. [Crossref] [PubMed]