Cost and effectiveness of video-assisted thoracoscopic surgery for clinical stage I non-small cell lung cancer: a population-based analysis
Introduction
Surgery is the cornerstone of curative treatment for early stage non-small cell lung cancer (NSCLC) (1). Lobectomy is the usual approach but the role of sublobectomy is also debated (1,2). Video-assisted thoracoscopic surgery (VATS) is a minimally invasive alternative to open thoracotomy and may lead to better survival outcome (1,3). Due to its minimally invasive nature, VATS is also associated with less surgical trauma, less use of narcotics, and fewer complications (4,5).
However, VATS is associated with a higher initial cost and may be overall more costly (6,7). Cost-effectiveness is an important issue nowadays (8). In an era when affordable cancer care is a worldwide issue, the cost-effectiveness of VATS should also be considered because this consideration will possibly affect patients’ access to cancer treatment (9).
To our knowledge, the cost-effectiveness (regarding cost per life year saved) of VATS has not been reported in the literature except our previous preliminary report (10). Therefore, the aim of our study is to compare the cost and effectiveness of VATS vs. conventional surgery (CS) for clinical stage I NSCLC via this updated population-based propensity score (PS) matched analysis.
Materials and methods
Data source
The Collaboration Center of Health Information Application (CCHIA) database is a set of databases with complete information regarding cancer and death registration, and reimbursement data from National Health Insurance (NHI) for the whole Taiwanese population. The cancer registry within CCHIA provides details regarding individual demographics, tumor histology, cancer primary sites, stage of disease, and primary surgical, radiation, and systemic therapy. NHI is a single compulsory payer with universal coverage in Taiwan and provides a comprehensive services package “All medically necessary services are covered. The package covers inpatient, outpatient, dental services, traditional Chinese medicine, and maintains a very long list of nearly 20,000 items of prescription drugs”. NHI’s reimbursement data files at the CCHIA provide information regarding the income of the insured, details of treatment received, and the characteristics of health care providers.
Study population and study design
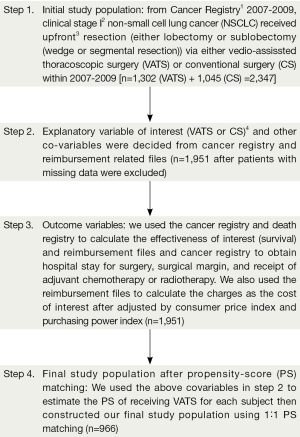
Our study flow chart is depicted in Figure 1. Our target populations were clinical stage I NSCLC patients received either VATS or CS within 2007-2009. In brief, the date of admission for surgery was used as the index date. We set the duration of interest as one year within the index date. We then decided the explanatory variable of interest (VATS vs. CS) based on the reimbursement coding. We also collected other covariables for the adjustment of potential non-randomized treatment selection and cost and effectiveness data from the CCHIA (see next sub-section “other explanatory covariables”). Finally, we constructed a PS matched sample based on PS estimated through the above covariables to compare the cost and effectiveness of VATS vs. CS within the duration of interest. In PS analysis, we modeled the use of VATS (vs. CS) as the dependent variable and the covariables as independent variables, and used non-conditional logistic regression to model the probability of receiving VATS as commonly used in the literatures (11,12). We then used the logit of the probability as the PS, as commonly used in the literature (12). This study had been approved by Research Ethics Committee in our institute [CMUH103-REC-005].
Other explanatory covariables
Firstly, we searched the literature regarding potential factors that might influence the cost of VATS. We used the following balanced search filters regarding costs or economics in the PubMed “[‘costs and cost analysis’ (MeSH) OR costs (Title/Abstract) OR cost effective* (Title/Abstract)] OR [cost* (Title/Abstract) OR ‘costs and cost analysis’ (MeSH:noexp) OR cost benefit analysis* (Title/Abstract) OR cost-benefit analysis (MeSH) OR health care costs (MeSH:noexp)]” as in the literatures (13,14). We combined the above keywords with “(cancer) AND (VATS OR thoracoscopic)” and found that social economic status (SES), surgeons’ case load, and tumor location might influence the cost after VATS (15-17). Secondly, we collected other factors that were not reported in the literature but that might affect the cost of VATS based on our clinical and research experiences. In this regard, we also included patient demographic factors (age, gender, and residency region), patient characteristics (comorbidity), disease characteristics (histology and pathological stage), treatment pattern (surgical type), and health service provider characteristics (treating hospital preference) based on our clinical experiences and prior NHI and CCHIA related studies (18-24). Age was classified as ≥65 years old or not. Patient residency was classified as northern Taiwan or elsewhere. SES was classified as high (income greater than minimal wage) or not. Histology was classified as adenocarcinoma or others. Pathological stage was classified as early or advanced (beyond stage I). Tumor location was classified as lower vs. upper/middle. Surgical type was classified as lobectomy or sublobectomy. Surgeons’ case load was classified as high vs. low (split at the median in our study sample). Treating hospital preference was classified as high (at least half of their patients were treated by VATS) or low.
Cost and effectiveness assessment
We included the following issues in effectiveness assessment: hospital stay for surgery, pathological stage, surgical margin, receipt of adjuvant chemotherapy or radiotherapy, survival within duration of interest, and overall survival. We obtained survival status according to the death registry, hospital stay from reimbursement files, and the other issues from cancer registry. The cost and cost-effectiveness were conducted from a Taiwan NHI perspective (i.e., charges to NHI). The cost was limited to the duration of interest then converted to 2013 USD by purchasing the power parity and consumer price indexes. The cost within our duration of interest was further broken down into four quarters (i.e., every 3 months) to illustrate the cost in different disease phrases. We then applied various thresholds of willingness-to-pay (WTP) to calculate the net benefit (NB) when VATS was compared to CS by applying the following equation (25):
WTP refers to the amount of money the payer is willing to pay for an outcome. The commonly cited WTP threshold [50,000-100,000 USD/life year (LY)] means that the payer is generally willing to pay 50,000-100,000 USD to gain a year of life and this was considered a threshold to decide whether an intervention was cost-effective or not (26,27). This WTP range also covers the World Health Organization (WHO) criteria (3 times gross domestic product per capita) regarding cost-effectiveness in Taiwan [around 58,042 (19,347.329*3)] (28). When the incremental NB (INB) of an intervention is positive at a specific WTP level, this means that this intervention is associated with a positive net monetary gain, so it is also cost-effective at this specific WTP level.
Statistical analysis
Tabulation and standardized difference were used to assess the balance of covariates between PS-matched groups. We used a stratified log-rank test to compare the survival of VATS versus CS for the entire follow-up period (censored on 1 January 2012) (12). Other outcomes between VATS and CS were compared with McNemar test or paired t-test (12). We used the paired t-test to evaluate the statistical significance of the INB, and then constructed the cost-effectiveness acceptability curve (CEAcC) (25). SAS 9.3 (SAS Institute, Cary, NC, USA) was used for all the analysis.
Results
Identification of the study cases (Figure 1, Table 1)
Full table
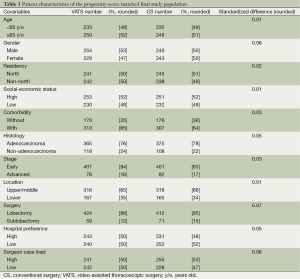
As revealed in Figure 1, 2,347 clinical stage I NSCLC patients treated with either VATS or CS were identified as the initial study population. After exclusion of those with missing data and matching by PS, the final study population included 966 patients. The characteristics of these patients are described in Table 1. A good balance of covariables and small standardized differences (<0.1) were seen for all covariables.
Cost and effectiveness
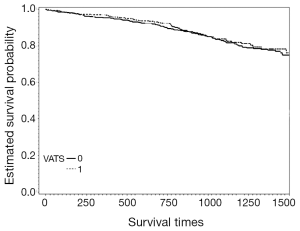
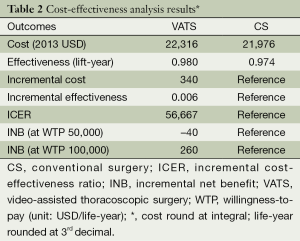
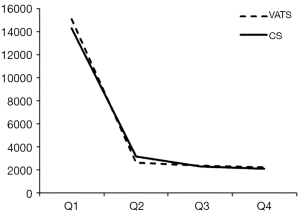
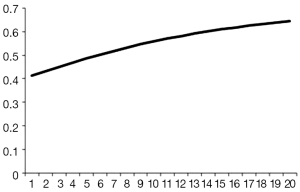
The mean hospital stay (in days, with standard deviation (SD)) were 14.4 [7] and 16.1 (7.7) for VATS and CS respectively (P=0.002). The distribution regarding surgical margin and receipt of adjuvant chemotherapy or radiotherapy were similar between VATS and CS without statistical significance. For the entire follow-up period, the survival rate of VATS was better than CS (2 years: 92% vs. 90%, P=0.8), but was not of statistical significance. The Kaplan-Meier survival curve is depicted in Figure 2. The mean cost (2013 USD) and survival (year) within one year after surgery were higher for VATS versus CS ($22,316 vs. $21,976; 0.98 vs. 0.974). Given the above incremental cost $340 (=22,316 – 21,976) and incremental effectiveness 0.06 (=0.98 – 0.974) LY, the net benefit if WTP equals $50,000/LY would be negative $40 (=0.006*50,000 – 340) but positive $260 (=0.006*100,000 – 340) if WTP equals $100,000/LY. The incremental cost-effectiveness ratio (ICER) when VATS was compared to CS was 56,667 (=340/0.006) (USD/LY). The above results were also tabulated in Table 2. Although VATS is associated with higher initial cost (in the 1st quarter after surgery), the difference is not obvious in the end of follow-up (Figure 3). When we changed the WTP level, the corresponding probability of VATS to be cost-effective (i.e., positive net benefit) as estimated by paired t-test was shown in Figure 4. For example, the probability was 0.49 & 0.56 at WTP 50,000 & 100,000, respectively.
Full table
Discussion
In this population-based propensity-score matched cost-effectiveness analysis, we provide the first empirical evidence that VATS is potentially cost-effective versus CS in the short-term (1 year) within the common WTP levels from a payer’s perspective since our estimated ICER ($56,667 USD/LY) was below either the common criteria ($100,000 USD/LY) or the WHO criteria ($58,042 USD/LY).
Our results were compatible with the literatures in that VATS provides better survival (1,3). The higher cost for VATS might partly be due to the higher operation fee for VATS vs. CS (dereferences in operation fee: $399 for VATS lobectomy vs. CS lobectomy whereas $224 for VATS sublobectomy vs. CS sublobectomy, both in USD 2013). Our estimated cost within 1 year after surgery was also higher for VATS as ever reported by other study (8), although conflict results had also been reported (7,15,29-31). Our updated estimates were also in line with our previous preliminary estimates but were more representative now given the much larger sample size (10).
Although the interpretation of our results is that VATS is potentially cost-effective within the common WTP levels in the short term, we could not specify to specific VATS due to data limitation. For example, we could not define whether VATS was complete or assisted (32). There were also some other limitations in our study. Firstly, there is always concern in potential unobserved confounding bias although we had performed comprehensive literature searching and used our own clinical and research experiences to include potential confounders we suspected. Secondly, although the long term outcome of early stage NSCLC was quite good, whether our duration of interest (1 year) was long enough to fully capture the cost-effectiveness of VATS versus CS might deserve further studies although longer follow-up might make VATS more favorable given the slightly improved survival and similar cost in the end of our follow-up period (as revealed in Figures 2,3).
Conclusions
In this population-based propensity-score matched cost-effectiveness analysis, we provide the first empirical evidence that when compared to CS, VATS was potentially cost-effective in the short term (1 year) within the common willingness-to-pay levels from a payer’s perspective in Taiwan. Further studies would be helpful to see the long term results and whether the same results could be obtained in other health care systems.
Acknowledgements
The data analyzed in this study was provided by the Collaboration Centre for Health Information Application (CCHIA), Ministry of Health and Welfare, Executive Yuan, Taiwan. The author would like to thank the funding agencies [the Ministry of Health and Welfare (MOHW104-TD-B-111-03)] for their financial support. The corresponding author would like to thank Dr. Ya-Chen Tina Shih for her mentoring.
Funding: This work was supported by the Health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence, Taiwan [MOHW104-TD-B-111-03].
Authors’ contributions: The authors—and not the funding bodies or CCHIA—had full control of in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication. All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript. C.R.C participated in the concept and design, analysis and interpretation of data, and drafting of the manuscript. H.Y.F, F.Y.H, H.C.H and Y.S.L participated in the concept and design, interpretation of data, and drafting of the manuscript. C.Y.C, S.H.S, P.R.C and C.K.C participated in the concept and design, and interpretation of data. All authors have approved the manuscript as submitted.
Disclosure: The authors declare no conflict of interest.
References
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi89-98. [PubMed]
- Fan J, Wang L, Jiang GN, et al. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol 2012;19:661-8. [PubMed]
- Li Z, Liu H, Li L. Video-assisted thoracoscopic surgery versus open lobectomy for stage I lung cancer: A meta-analysis of long-term outcomes. Exp Ther Med 2012;3:886-892. [PubMed]
- Chen HW, Du M. Video-assisted thoracic surgery for lung carcinoma. J Thorac Dis 2013;5:912-3. [PubMed]
- Fang HY, Chen CY, Wang YC, et al. Consistently lower narcotics consumption after video-assisted thoracoscopic surgery for early stage non-small cell lung cancer when compared to open surgery: a one-year follow-up study. Eur J Cardiothorac Surg 2013;43:783-6. [PubMed]
- Van Schil P. Cost analysis of video-assisted thoracic surgery versus thoracotomy: critical review. Eur Respir J 2003;22:735-8. [PubMed]
- Piwkowski C, Gabryel P, Gałęcki B, et al. High costs as a slow down factor of thoracoscopic lobectomy development in Poland - an institutional experience. Wideochir Inne Tech Malo Inwazyjne 2013;8:334-41. [PubMed]
- Abbott DE, Sutton JM, Edwards MJ. Making the case for cost-effectiveness research. J Surg Oncol 2014;109:509-15. [PubMed]
- Chalkidou K, Marquez P, Dhillon PK, et al. Evidence-informed frameworks for cost-effective cancer care and prevention in low, middle, and high-income countries. Lancet Oncol 2014;15:e119-31. [PubMed]
- Chien CR, Wang PH, Shih YCT. Cost-effectiveness analysis of video-assisted thoracic surgery vs. open surgery for patients with newly diagnosed stage I nonsmall cell lung cancer. ISPOR 17th Annual International Meeting, Washington DC, USA; 2012 Jun 2-6. Value Health 2012;15:A77.
- Rosenbaum PR. Basic Tools of Multivariate Matching. In: Rosenbaum PR. eds. Design of Observational Studies. Springer Series in Statistics, Springer, 2010:163-85.
- Austin PC, Chiu M, Ko DT, et al. Propensity score matching for estimating treatment effect. In: Faries DE, Leon AC, Haro JM, et al. eds. Analysis of Observational Health Care Data Using SAS. USA: SAS Institute, Cary, NC, 2010:51-84.
- Glanville J, Paisley S. Identifying economic evaluations for health technology assessment. Int J Technol Assess Health Care 2010;26:436-40. [PubMed]
- Smieliauskas F, Chien CR, Shen C, et al. Cost-effectiveness analyses of targeted oral anti-cancer drugs: a systematic review. Pharmacoeconomics 2014;32:651-80. [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [PubMed]
- Cho S, Do YW, Lee EB. Comparison of costs for video-assisted thoracic surgery lobectomy and open lobectomy for non-small cell lung cancer. Surg Endosc 2011;25:1054-61. [PubMed]
- Gopaldas RR, Bakaeen FG, Dao TK, et al. Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients. Ann Thorac Surg 2010;89:1563-70. [PubMed]
- Lin CC, Hsia TC, Chien CR. 3rd line Erlotinib for lung cancer in Asia may be as cost-effective as in the Western world. Lung Cancer 2012;76:499-500. [PubMed]
- Chien CR, Su SY, Cohen L, et al. Use of Chinese medicine among survivors of nasopharyngeal carcinoma in Taiwan: a population-based study. Integr Cancer Ther 2012;11:221-31. [PubMed]
- Hsia TC, Tu CY, Chen HJ, et al. Effectiveness of intensity-modulated radiotherapy for lung cancer. Clin Oncol (R Coll Radiol) 2013;25:447-8. [PubMed]
- Chien CR, Shih YC. Use of personalized decision analysis in decision making for Palliative vs. surgical management of the oldest-old patients with localized skin cancer in a culturally sensitive environment: a case study of a 96-year-old male Taiwanese patient. J Pain Symptom Manage 2013;45:792-7. [PubMed]
- Chien CR, Lin HW, Yang CH, et al. High case volume of radiation oncologists is associated with better survival of nasopharyngeal carcinoma patients treated with radiotherapy: a multifactorial cohort analysis. Clin Otolaryngol 2011;36:558-65. [PubMed]
- Chien CR, Hsia TC, Chen CY. Cost-effectiveness of chemotherapy combined with thoracic radiotherapy versus chemotherapy alone for limited stage small cell lung cancer: A population-based propensity-score matched analysis. Thoracic Cancer 2014;5:530-6.
- Ke TW, Liao YM, Chiang HC, et al. Effectiveness of neoadjuvant concurrent chemoradiotherapy versus up-front proctectomy in clinical stage II-III rectal cancer: A population-based study. Asia Pac J Clin Oncol 2014. [Epub ahead of print]. [PubMed]
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. [PubMed]
- Konski A. Economic analysis of health care interventions. Semin Radiat Oncol 2008;18:168-74. [PubMed]
- Shih YC, Halpern MT. Economic evaluations of medical care interventions for cancer patients: how, why, and what does it mean? CA Cancer J Clin 2008;58:231-44. [PubMed]
- Available online: http://www.who.int/choice/costs/CER_thresholds/en/
- Farjah F, Backhus LM, Varghese TK, et al. Ninety-day costs of video-assisted thoracic surgery versus open lobectomy for lung cancer. Ann Thorac Surg 2014;98:191-6. [PubMed]
- Lacin T, Swanson S. Current costs of video-assisted thoracic surgery (VATS) lobectomy. J Thorac Dis 2013;5 Suppl 3:S190-3. [PubMed]
- Ramos R, Masuet C, Gossot D. Lobectomy for early-stage lung carcinoma: a cost analysis of full thoracoscopy versus posterolateral thoracotomy. Surg Endosc 2012;26:431-7. [PubMed]
- He J, Shao W, Cao C, et al. Long-term outcome and cost-effectiveness of complete versus assisted video-assisted thoracic surgery for non-small cell lung cancer. J Surg Oncol 2011;104:162-8. [PubMed]


