
Inter-tumor heterogeneity of PD-L1 expression in non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide (1-3). However, there have been no marked improvements in the long-term survival of patients with advanced non-small cell lung cancer (NSCLC) over the past couple of decades (4). Nonetheless, following advances in research on immune checkpoint mechanisms (5-9), programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1)-targeted therapies have been rapidly developed, and several clinical trials have shown their antitumor effects with remarkable responses in patients with NSCLC (10-13). Based on results of these clinical trials, some immune checkpoint inhibitors have been recommended as standard treatments according to the therapeutic algorithm included in the current guidelines for primary lung cancer treatment (14-16). Nowadays, some monoclonal antibodies (pembrolizumab, nivolumab, and atezolizumab) are available in clinical practice for use as second-line treatments in patients with both squamous and non-squamous NSCLC. However, only pembrolizumab can be used as the first-line treatment in patients exhibiting high PD-L1 expression (17).
At the time of approval by the Food and Drug Administration (FDA), the 22C3 antibody clone used in the PD-L1 immunohistochemistry (IHC) assay was approved as a companion diagnostic antibody for pembrolizumab, whereas the 28-8 clone was approved as a complementary diagnostic antibody for nivolumab and atezolizumab to determine the eligibility of patients with NSCLC (18,19). The Blueprint Project Team has recently demonstrated that all 3 PD-L1 IHC assays (22C3, 28-8, and SP263) are interchangeable; thus, use of any of these is anticipated as a companion or complementary diagnostic test in the future (20). Furthermore, other research groups have investigated and reported on the PD-L1 staining heterogeneity shown between large and small specimens from the same tumor (intra-tumor heterogeneity) (21-23). However, there have also been a few reports regarding the heterogeneity observed between primary and metastatic tumors in the same patient (inter-tumor heterogeneity).
In this study, we aimed to explore inter-tumor heterogeneity between primary and secondary tumors (paired metastatic lymph nodes) obtained from the same patient with NSCLC using PD-L1 IHC 22C3 and 28-8 antibodies (Dako, Glostrup, Denmark). Moreover, we assessed the interchangeability of 22C3 and 28-8 IHC staining in both primary tumors and lymph nodes.
Methods
Subjects and tumor samples
Between December 1, 2014 and May 7, 2018, 517 patients with NSCLC underwent surgical resection at the Saitama Cardiovascular and Respiratory Center, including 45 (8.5%) pathological N1 tumors, 50 (9.5%) N2 tumors, and 1 (0.18%) N3 tumor. In this study, the inclusion criteria were that (I) surgery cases with lymph node dissections, (II) histologically NSCLC, (III) pathologically nodal positive, and (IV) informed consent. However, we excluded all cases with insufficient volume for PD-L1 testing, and did not include any biopsy samples. Finally, only 35 (6.8%) were available for immunohistochemical staining using the PD-L1 22C3 and 28-8 antibodies. Pathological diagnosis and tumor proportion score (TPS) were determined by an expert pathologist (Y Shimizu) based on the WHO classification version 8 and IASLC Atlas of PD-L1 Testing in Lung Cancer (24,25). Written informed consent for medical research was obtained from all patients. Clinical and pathological data were stored in a database in accordance with hospital confidentiality and privacy rules. The study protocol was approved by the institutional review board of Saitama Cardiovascular and Respiratory Center (2018005).
PD-L1 IHC using the 22C3 and 28-8 antibodies
PD-L1 IHC 22C3 and 28-8 pharmDx antibodies (Dako, Glostrup, Denmark) were used in this study. The assays were performed using the Dako Autostainer Link 48 system (Dako, Glostrup, Denmark) employing an automated staining protocol (26,27). According to the manufacturer’s instructions, all block specimens of formalin-fixed, paraffin-embedded tissues were sectioned at a thickness of 4–5 µm. After deparaffinization, sections were incubated for 20 min at 97 °C in the Autostainer slide rack, and then stored in EnVision FLEX Wash Buffer (Dako, Glostrup, Denmark) for 5 min at room temperature. Finally, according to each manufactures’ instructions, all staining and counterstaining were performed along with a positive control and a negative antibody control (buffer, no primary antibody).
TPS
PD-L1 expression was evaluated only in tumor cells from primary tumors and paired metastatic lymph nodes (Figure 1). A tumor cell was defined as positive for PD-L1 staining, whenever any partial or complete membranous staining was detected (note: limited cytoplasmic only staining did not indicate positive staining). At least 100 viable tumor cells were required to judge PD-L1 IHC positivity on a slide. The pathologist evaluated all slides at 10×, 20×, and 40× magnifications. TPS was defined as the percentage of positive viable tumor cells among all viable tumor cells evaluated (TPS = positive tumor cells/positive plus negative tumor cells). According to TPS expression levels, 3 subgroups were created: (I) no expression (<1%), (II) low expression (1–49%), and (III) high expression (≥50%).
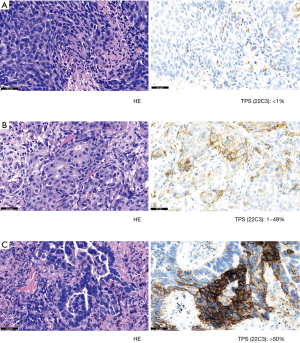
Statistical analysis
All statistical analyses were conducted using SPSS 19.0 software (SPSS, Chicago, IL, USA), and a P value of <0.05 was considered significant. Pearson’s correlation was used to evaluate the correlation coefficient between primary tumors and paired metastatic lymph nodes, and between the 22C3 and 28-8 antibodies.
Results
Patient characteristics
Mean age of the 35 patients was 66.8 years (43–83 years) (Table 1). The majority of patients were male (n=25, 71.4%), and 25 were smokers (71.4%). Of the 35 patients, 27 (77.1%) were diagnosed with adenocarcinoma and 6 (17.1%) with squamous cell carcinoma. In total, ten patients exhibited pathological N1 tumors (28.6%), whereas the remaining patients featured N2 or N3 tumors. Overall, 11 (31.4%) tumors were epidermal growth factor receptor (EGFR) mutation-positive, and only 1 (2.9%) tumor was anaplastic lymphoma kinase (ALK) fusion gene-positive and EGFR mutation-negative. TPS for both primary tumors and metastatic lymph nodes is presented in Table 2. Although the subgroup distribution for TPS was similar between the 22C3 and 28-8 antibodies, there were differences exhibited between primary tumors and paired lymph nodes (P<0.001 and P<0.001, respectively).

Full table

Full table
TPS for Dako 22C3 and 28-8 pharmDx
The distribution of TPS categories was very similar between the 22C3 and 28-8 antibodies in both primary tumors (Figure 2A,B) and metastatic lymph nodes (Figure 2C,D). Conversely, TPS values were different between primary tumors and lymph nodes in both the 22C3 (Figure 2A,C) and 28-8 assays (Figure 2B,D). Table 2 demonstrated each concordant rate of TPS between primary tumor and lymph node. TPS values in primary tumors using the 22C3 and 28-8 antibodies were about 20% in the no expression, 60% in the low expression, and 20% in the high expression groups. In contrast, in metastatic lymph nodes, proportion score values were approximately 50% in the no expression, 35% in the low expression, and 15% in the high expression groups.
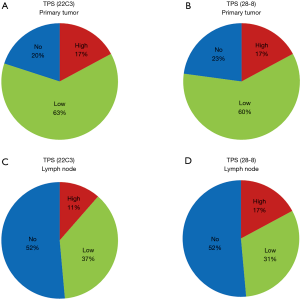
Interrelationship of TPS between Dako 22C3 and 28-8 pharmDx
In the PD-L1 IHC 22C3 assay, 26 primary tumors showed the same TPS as that of their paired metastatic lymph nodes (Table 3), classifying 5 tumors (14.3%) in the no expression, 17 tumors (48.6%) in the low expression, and 4 tumors (11.4%) in the high expression groups. Conversely, in the PD-L1 IHC 28-8 assay, 25 primary tumors showed the same TPS as that of their paired metastatic lymph nodes, classifying 14 tumors (40.0%) in the no expression, 7 tumors (20.0%) in the low expression, and 4 tumors (11.4%) in the high expression groups (Table 4). TPS concordance rates were 74.2% (primary tumors) and 71.4% (lymph nodes) between the two assays (22C3 and 28-8 antibodies). In addition, Pearson’s chi-square test revealed a significant difference between the performed assays (P<0.001 for 22C3 and P<0.001 for 28-8).
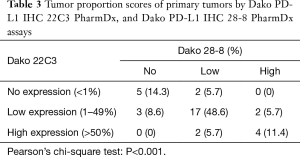
Full table
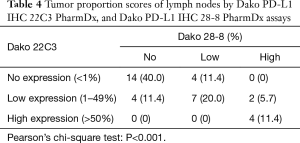
Full table
Interrelationship of TPS between primary tumors and lymph nodes
When we performed the PD-L1 IHC 22C3 assay, ten primary tumors showed the same TPS as that of their paired metastatic lymph nodes, classifying 4 (11.4%) in the no expression and 6 (17.1%) in the low expression groups (Table 5). Surprisingly, there was no case categorized in the high expression group for neither the ten primary tumors nor their paired metastatic lymph nodes. The concordance rate between the ten primary tumors and their paired lymph nodes was 28.6%, and the chi-square test revealed no significant differences between the performed assays (P=0.300).
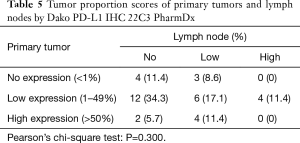
Full table
Concurrently, when the PD-L1 IHC 28-8 assay was performed, 11 primary tumors showed the same TPS as that of their paired metastatic lymph nodes, classifying 4 (11.4%) in the no expression, 6 (17.1%) in the low expression, and 1 (2.9%) in the high expression groups (Table 6). The calculated concordance rate was 31.4% (P=0.989).
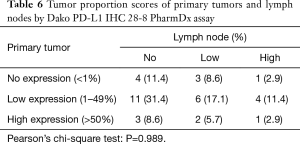
Full table
Interrelationship of PD-L1 expression
The percentage of PD-L1 positive stains for each case is shown in Figure 3A. Regarding PD-L1 expression in primary tumors, the fitting curves of both scatter plots for the two distinct PD-L1 assays were similar, exhibiting a high correlation coefficient (Figure 3B). In the case of the metastatic lymph nodes, very similar fitting curves and a high correlation coefficient were also observed (Figure 3C). Conversely, PD-L1 expression distribution among tumor tissues was rather different, indicating a discordance between primary tumors and paired metastatic lymph nodes for both the 22C3 and the 28-8 IHC assays (Figure 3D,E).
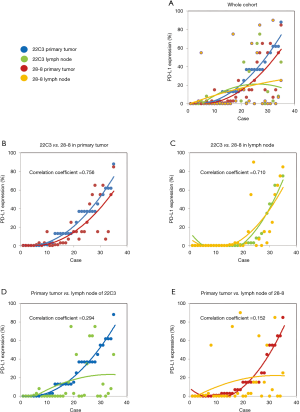
Discussion
In recent years, tumor profiling by genotyping and IHC along with histological diagnosis of primary lung cancer, which have already become common in clinical practice, have led to a dramatic increase in the roles of pulmonary pathologists. According to the National Comprehensive Cancer Network guidelines (15), NSCLC is classified based on genomic alterations [in EGFR, ALK, ROS proto-oncogene 1 (ROS1), and B-Raf proto-oncogene (BRAF)] or immunological markers (PD-L1 IHC). Based on results of a phase III investigation, pembrolizumab was approved for use in patients with tumors showing PD-L1 expression >50% (11), and the PD-L1 IHC 22C3 (Dako) assay was approved by both the US FDA and the European Medicines Agency as a companion diagnostic test to determine patient treatment eligibility. Therefore, demand for precision testing has increased in both laboratories and hospitals.
In 2018, Tsao et al. investigated the interchangeability of some PD-L1 antibodies (20). Among the five antibody assays (22C3, 28-8, SP142, SP264, and 73-10) they evaluated, three antibodies (22C3, 28-8, and SP263) were found to be compatible with one another for determining TPS of primary lung cancer cells. Furthermore, Humphries et al. have demonstrated high concordance between the 22C3 and SP263 IHC assays in NSCLC (28). In the future, the use of any one of these interchangeable antibodies is anticipated to aid treatment decision making. However, the best site for evaluating PD-L1 expression in a patient’s body remains unidentified.
Heterogeneity is an important issue in the field of oncology, and has been that way for some time (29). Heterogeneity as a concept covers both inter- and intra-patient variability among organs, tissues, cells, and molecules (30). Clinical discussions often involve the site that can be or should be biopsied to determine treatment. Although there are published studies regarding intra-tumor, inter-observer, or inter-assay heterogeneities, there are very few reports on inter-tumor heterogeneity of PD-L1 expression in NSCLC. Sakakibara et al. have reported good concordance of PD-L1 IHC assay between primary tumors and their paired lymph node metastases in NSCLC (r=0.93; P=0.02); however, their patient cohort was very small (n=5), and non-clinically used antibodies (rabbit monoclonal, clone EPR1161) were implemented in their study (31). We explored differences in PD-L1 expression between primary tumors and their paired lymph node metastases in NSCLC, using the Dako PD-L1 IHC 22C3 and 28-8 pharmDx assays. In our study, while inter-assay heterogeneity was tolerable (74.2% in primary tumors and 71.4% in metastatic lymph nodes), inter-tumor heterogeneity was non-negligible due to the low TPS concordance rate observed (28.6% in the 22C3 assay and 31.4% in the 28-8 assay). Results of PD-L1 expression analysis and correlation coefficients were comparable (Figure 3).
According to the current guidelines, PD-L1 staining of tumor cells is a pivotal examination. However, inter-tumor heterogeneity remains an unsolved clinical problem, which can potentially preclude precise treatment planning for patients with advanced NSCLC. Here, we present a representative case, which may raise controversies among oncologists (Figure 4). In this case, both the 22C3 and 28-8 IHC assays showed positive staining in primary tumors but no staining in paired metastatic lymph nodes. These findings indicate that chemotherapy or monotherapy with pembrolizumab should be recommended for patients with advanced NSCLC when referring to the result of PD-L1 staining in primary tumors. However, these medications should not be administered when lymph node staining is referred. Currently, the ideal site for immunotherapy biomarker remains unknown. This issue must be addressed by future clinical studies that will ideally include a greater number of patients.
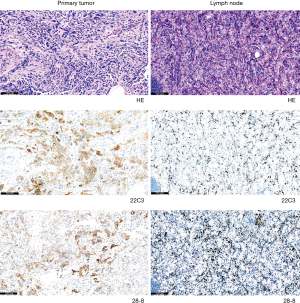
This study had several limitations: (I) the relatively small number of cases evaluated; (II) the single center nature of the study; and (III) the surgical resection of all samples. It should be noted that obtaining sufficient specimen volume for PD-L1 IHC assay from both primary tumors and any other metastatic tissues recovered from a patient with or without surgical indications is very difficult in clinical settings. In the future, multicenter prospective studies may elucidate the association between inter-tumor heterogeneity and immunotherapy treatment outcomes.
In summary, we observed a favorable similarity between the detection capabilities of both 22C3 and 28-8 IHC assays and a serious discrepancy in PD-L1 staining between primary and secondary (metastatic) NSCLC tumors. The interchangeability of these two assays should make it easier to select one assay based on cost concerns; however, inter-tumor heterogeneity may make it difficult to treat patients efficiently. We believe our present study will positively impact future clinical studies regarding the ideal site for testing PD-L1 expression.
Acknowledgments
We thank Mr. Hidehiro Numagami, Mr. Yasuhito Kobayashi and Mei Miyagawa for excellent technical assistance.
Funding: This work was supported by grants from Saitama Cardiovascular and Respiratory Center (grant numbers: 17ET and 18ET).
Footnote
Conflicts of Interest: The authors have no conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board of the Saitama Cardiovascular and Respiratory Center (approval numbers: 2018005). Written informed consent was obtained from all patients to participate in this study, and this study outcome will not affect the future management of the patients.
References
- Malvezzi M, Carioli G, Bertuccio P, et al. European cancer mortality predictions for the year 2017, with focus on lung cancer. Ann Oncol 2017;28:1117-23. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Hori M, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2009: A study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2015;45:884-91. [Crossref] [PubMed]
- Pilkington G, Boland A, Brown T, et al. A systematic review of the clinical effectiveness of fi rst-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer. Thorax 2015;70:359-67. [Crossref] [PubMed]
- Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor – Deficient Mice. Science 2001;291:319-22. [Crossref] [PubMed]
- Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci 2002;99:12293-7. [Crossref] [PubMed]
- Leach DR, Krummel MF, Allison JP. Enhancement of Antitumor Immunity by CTLA-4 blockade. Science 1996;271:1734-6. [Crossref] [PubMed]
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887-95. [Crossref] [PubMed]
- Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol 2006;27:195-201. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Brahmer JR, Rodríguez-Abreu D, Robinson AG, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol 2017;18:1600-9. [Crossref] [PubMed]
- Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959-65. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- The Japan Lung Cancer Society. Guidelines for Diagnosis ans Treatment of the Lung Cancer 2018. Available online: (28 July 2019, date last accessed).https://www.haigan.gr.jp/modules/guideline/index.php?content_id=3
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:863-70. [Crossref] [PubMed]
- Brahmer JR, Govindan R, Anders RA, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer 2018;6:75. [Crossref] [PubMed]
- Büttner R, Gosney JR, Skov BG, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3867-76. [Crossref] [PubMed]
- Yu H, Boyle TA, Zhou C. PD-L1 Expression in Lung Cancer. J Thorac Oncol 2016;11:964-75. [Crossref] [PubMed]
- Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 2018;13:1302-11. [Crossref] [PubMed]
- Munari E, Zamboni G, Lunardi G, et al. PD-L1 Expression Heterogeneity in Non–Small Cell Lung Cancer: Defining Criteria for Harmonization between Biopsy Specimens and Whole Sections. J Thorac Oncol 2018;13:1113-20. [Crossref] [PubMed]
- Bassanelli M, Sioletic S, Martini M, et al. Heterogeneity of PD-L1 Expression and Relationship with Biology of NSCLC. Anticancer Res 2018;38:3789-96. [Crossref] [PubMed]
- Li C, Huang C, Mok TS, et al. Comparison of 22C3 PD-L1 Expression between Surgically Resected Specimens and Paired Tissue Microarrays in Non–Small Cell Lung Cancer. J Thorac Oncol 2017;12:1536-43. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- International Association for the Lung Cancer. IASLC Atlas of PD-L1 Testing in Lung Cancer. Available online: https://www.iaslc.org/Portals/0/iaslc_pd-l1_atlas_mar2018_lo-res.pdf?ver=2019-06-06-153849-143
- Dako. PD-L1 IHC 22C3 pharmDx: Non-Small Cell Lung Cancer [interpretation manual]. Accessed 28 July 2019. Available online: https://www.agilent.com/cs/library/usermanuals/public/29158_pd-l1-ihc-22C3-pharmdx-nsclc-interpretation-manual.pdf
- Dako/Agilent. PD-L1 IHC 28-8 pharmDx: Interpretation Manual (Non-Squamous Non-Small Cell Lung Cancer). Accessed28 July 2019. Available online: https://www.agilent.com/cs/library/usermanuals/public/29126_pd-l1-28-8-nsclc-interpretation-manual_row.pdf
- Humphries MP, McQuaid S, Craig SG, et al. Critical Appraisal of Programmed Death Ligand 1 Reflex Diagnostic Testing: Current Standards and Future Opportunities. J Thorac Oncol 2019;14:45-53. [Crossref] [PubMed]
- Spremulli EN, Dexter DL. Human tumor cell heterogeneity and metastasis. J Clin Oncol 1983;1:496-509. [Crossref] [PubMed]
- Allison KH, Sledge GW Jr. Heterogeneity and Cancer. Oncology (Williston Park) 2014;28:772-8. [PubMed]
- Sakakibara R, Inamura K, Tambo Y, et al. EBUS-TBNA as a Promising Method for the Evaluation of Tumor PD-L1 Expression in Lung Cancer. Clin Lung Cancer 2017;18:527-534.e1. [Crossref] [PubMed]


