Coinfection of Strongyloides stercoralis and Aspergillus found in bronchoalveolar lavage fluid from a patient with stubborn pulmonary symptoms
Introduction
Strongyloides stercoralis (S. stercoralis) is a facultative parasite that is endemic in tropical and subtropical regions and occurs sporadically in temperate areas (1). Adult worms live in the host’s small intestine, such as humans, dogs, cats, etc. The larvae can invade the lung, brain, liver, and kidney, as well as other tissues or organs, causing strongyloidiasis. The clinical spectrum of strongyloidiasis varies from asymptomatic infection and mild symptomatic abdominal and skin diseases to fatal disseminated infection in immunosuppressed patients (2). Pulmonary strongyloidiasis is one of the most important signs of disseminated strongyloidiasis.
Invasive pulmonary aspergillosis is a severe fungal disease and can be found in immunocompromised patients (3). Its mortality rate is 58-88.1% and almost 100% without treatment (4). Symptoms are nonspecific: cough, sputum production, and dyspnea.
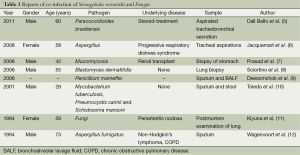
Immunocompromised patients can contract strongyloidiasis and fungal disease, but coinfection with S. stercoralis and a fungus has been rarely reported, even in immunocompromised patients. Herein, we reviewed all the S. stercoralis and fungal coinfection cases that we could find in PubMed (5-12) (Table 1); eight cases were found. Only two cases had coinfection with S. stercoralis and Aspergillus (7,12), while seven cases had fungal coinfections detected in the respiratory system.
Full table
Case presentation
A 74-year-old male had a 3-month history of cough, expectoration, and shortness of breath after activity. Two weeks before, he presented with hemoptysis, accompanied by fever, a poor appetite, and an obvious weight loss, but without chills, night sweats, or chest pain. Aspergillus was discovered in several sputum cultures, so he was administered an antifungal treatment (voriconazole, 0.2 g, twice daily) for 2 weeks; however, the treatment effect was not ideal, and his hemoptysis did not disappear. For further examination and treatment, he was transferred to a local hospital.
On admission, he had an increased C-reactive protein level of 110 (normal, <10) mg/L, a white blood cell count of 11.34 (normal, 4.0-10.0) ×109/L, a neutrophil percentage of 90.6% (normal, 50-70%), and an erythrocyte sedimentation rate of 96 (normal, <15) mm/h but had a decreased eosinophil percentage of 0.1% (normal, 0.5-5%). Coagulation-related tests and his platelet count were normal; therefore, hemoptysis caused by coagulation dysfunction was not considered. Tuberculosis and heart failure were excluded with a related examination.
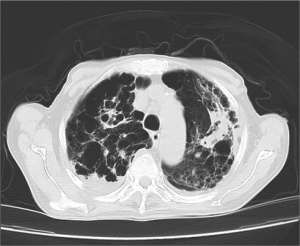
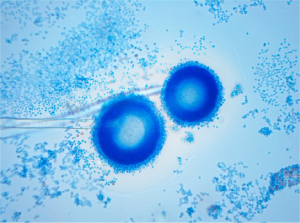
In addition to chronic obstructive pulmonary disease (COPD)-like computed tomography (CT) imaging changes, multiple cystic and nodular lesions were visualized, especially in the right superior lobe (Figure 1). A bronchoscopy was carried out, and purulent plugs were found in the right superior lobe. There was no apparent evidence of lung cancer or bronchiectasia with either a CT or bronchoscopy. Aspergillus was also found in his bronchoalveolar lavage fluid (BALF) culture (Figure 2).
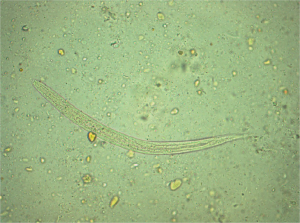
To our surprise, during a routine BALF examination, larvae of S. stercoralis were found in two of the six BALF smear slides (Figure 3). Larvae were also found in his feces (Figure 4). These larvae had a round tip and tapering tail; were about 0.20-0.35 mm, with a double-ball pharyngeal tube; and had reproductive primordial located at the worm’s rear.
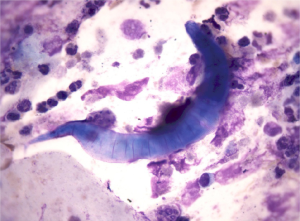
Upon further questioning of the patient, the patient stated that he had a habit of eating inadequately cooked snails. A year ago, the patient had acid reflux symptoms and abdominal pain. A pancreatic mass was demonstrated by endoscopic ultrasonography; autoimmune pancreatitis (AIP) was considered after excluding cancer. Prednisone (30 mg daily) was administered for 6 months.
Considering that antifungal therapy alone had a limited effect on symptom relief, the final diagnosis of coinfection with S. stercoralis and Aspergillus-induced pneumonia was established. After 8 weeks of albendazole and voriconazole treatment, his symptoms were relieved, and his hemoptysis disappeared.
Discussion
In recent years, Aspergillosis has been increasing: those patients treated with steroids and broad-spectrum antibiotics; ciliary impairment; and alveolar macrophage and neutrophil inhibition play a role in the development of fungal infection. Because of common, good hygienic conditions, we used to ignore parasites as a source of infection. Physicians usually consider symptoms that persist after antifungal treatment attributable to the sensitivity of the drug and patients’ immune function. Given certain clinical clues, physicians should think of the possibility of parasitic coinfections, because of the significance of treatment. This case reminds us to consider coinfection with S. stercoralis and Aspergillus that has been rarely reported.
The larvae of S. stercoralis that were found in the patient’s BALF and feces provide diagnostic proof of disseminated strongyloidiasis; however, the decreased eosinophil count remained elusive, because most parasitic diseases are accompanied by an increased number of eosinophils. Boulware et al. reported that about 84% of strongyloidiasis cases have an eosinophil percentage of >5% (13). The other patients, especially immunocompromised patients with disseminated strongyloidiasis who have a secondary response to S. stercoralis, may have a normal or reduced eosinophil count, as this case did. Eosinophils and neutrophils contribute to larval killing during the primary immune response, and neutrophils are effector cells in the secondary response to S. stercoralis (14). Thus, we could not exclude a parasitic infection with a normal or reduced eosinophil count.
The mortality rate for disseminated strongyloidiasis is as high as 77% among compromised hosts (15). The patients had been diagnosed with strongyloidiasis for an average of 56 months (intraquartile range, 4-72 months) after immigration (13). Early detection of parasitic infections in such individuals is extremely important, as disseminated strongyloidiasis is potentially fatal. Six months ago, AIP was considered in this patient because of abdominal pain, endoscopic ultrasonography changes in the pancreas, and increased IgG4. However, we also found increased eosinophils of 9.1% (normal, 0.5-5%) and IgE6740 (normal, <150 IU/mL); furthermore, continuous antigenic stimulation of chronic strongyloidiasis may also result in an enhanced IgG4 subclass response (16). As there was no presence or absence of pathogenic proof, whether his abdominal symptoms were connected with an S. stercoralis infection was unknown.
In conclusion, this case reminds us of the possibility of coinfection with S. stercoralis and Aspergillus in patients with an immunodeficiency. We also highlight that a normal or reduced eosinophil count does not exclude a parasitic infection. Early detection of a parasitic infection is important, as disseminated strongyloidiasis is potentially fatal. In this case, we experienced both chance and a challenge in determining the early diagnosis of strongyloidiasis.
Acknowledgements
This research was supported by grants from 973 Foundation (2013CB531606), National Science Foundation of China (81273282), Shanghai Municipal Commission for Science and Technology (11JC1410902), Changhai Hospital (CH125530300), Grant of Nanjing District (12MA056), The Educational reform fund of SMMU (jgc2013023).
Disclosure: The authors declare no conflict of interest.
References
- M Olmos J, Gracia S, Villoria F, et al. Disseminated strongyloidiasis in a patient with acquired immunodeficiency syndrome. Eur J Intern Med 2004;15:529-30. [PubMed]
- Uparanukraw P, Phongsri S, Morakote N. Fluctuations of larval excretion in Strongyloides stercoralis infection. Am J Trop Med Hyg 1999;60:967-73. [PubMed]
- Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev 2011;20:156-74. [PubMed]
- Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis 2001;32:358-66. [PubMed]
- Dall Bello AG, Severo CB, Oliveira Fde M, et al. Disseminated paracoccidioidomycosis (simulating metastatic lung cancer) and Strongyloides stercoralis hyperinfestation in a steroid-treated patient. J Clin Microbiol 2011;49:2054-5. [PubMed]
- Jacquemart C, Firre E, Thiry A, et al. Allergic bronchopulmonary aspergillosis associated with strongyloidiasis. Rev Med Liege 2008;63:469-73. [PubMed]
- Prasad N, Ram R, Satti Reddy V, et al. Non-fatal gastric mucormycosis in a renal transplant patient and review of the literature. Transpl Infect Dis 2006;8:237-41. [PubMed]
- Sciortino C, Omar R, De La Cruz R. Mixed pulmonary infection with Strongyloides stercoralis and Blastomyces dermatitidis. J Clin Microbiol 2006;44:4270-2. [PubMed]
- Deesomchok A, Tanprawate S. A 12-case series of Penicillium marneffei pneumonia. J Med Assoc Thai 2006;89:441-7. [PubMed]
- Toledo AC Jr, de Castro MR. Pneumocystis carinii pneumonia, pulmonary tuberculosis and visceral leishmaniasis in an adult HIV negative patient. Braz J Infect Dis 2001;5:154-7. [PubMed]
- Kiyuna M, Toda T, Tamamoto T, et al. An autopsy case of periarteritis nodosa associated with disseminated strongyloidiasis. Rinsho Byori 1994;42:883-7. [PubMed]
- Wagenvoort JH, Houben HG, Boonstra GL, et al. Pulmonary superinfection with Strongyloides stercoralis in an immunocompromised retired coal miner. Eur J Clin Microbiol Infect Dis 1994;13:518-9. [PubMed]
- Boulware DR, Stauffer WM, Hendel-Paterson BR, et al. Maltreatment of Strongyloides infection: case series and worldwide physicians-in-training survey. Am J Med 2007;120:545.e1-8.
- O’Connell AE, Hess JA, Santiago GA, et al. Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect Immun 2011;79:2770-8. [PubMed]
- Namisato S, Motomura K, Haranaga S, et al. Pulmonary strongyloidiasis in a patient receiving prednisolone therapy. Intern Med 2004;43:731-6. [PubMed]
- Genta RM, Lillibridge JP. Prominence of IgG4 antibodies in the human responses to Strongyloides stercoralis infection. J Infect Dis 1989;160:692-9. [PubMed]