
Melatonin for the treatment of sepsis: the scientific rationale
Introduction
Melatonin is an ancient biological compound sharing amino acid sequence homology with the melatonin present in cyanobacteria (1). It has been suggested melatonin was sequestered by eukaryotes as part of the endosymbiotic theory of evolution and has acquired roles in immunity, as a free radical scavenger and as the master hormonal regulator of the circadian rhythm (2). The circadian rhythm is a ubiquitous evolutionary homeostatic mechanism which acts as a biological clock to guide the differential release and regulation of hormones and to rhythmically alter the expression and translation of thousands of genes (3). It is comprised of complex, interacting, intrinsic cellular circadian clocks, the extrinsic daylight and fasting-feeding cycles and the release of hormonal regulators such as melatonin (4). Melatonin is a polypeptide derived from tryptophan, the synthesis and release of which is primarily governed by the pineal gland. Its release shows marked intra individual stability, but significant interindividual variability (5). Melatonin displays, however, a range of actions in different organs of the body, through its anti-inflammatory properties, as an oxidant scavenger and as an enhancer of the immune system. With a profoundly safe side-effect profile, melatonin has become a promising focus for research in critically ill patients. Exogenous administration of melatonin has been evaluated for its immunostimulatory, antiinflammatory and antioxidant properties and well as its effects on improvement of sleep cycling and architecture.
Chemistry
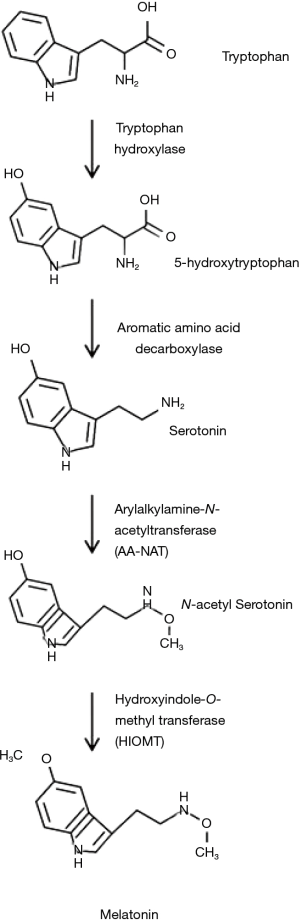
Melatonin is mainly produced within the pineal gland, but extrapineal sources of melatonin include the retina, platelets, skin, lymphocytes, bone marrow cells, cerebellum and the gastrointestinal tract (6,7). N-Acetyl-5-methoxytryptamine is synthesized from L-tryptophan via hydroxylation of the indole ring by tryptophan hydroxylase to produce 5-hydroxytryptophan (5-HTP). 5-HTP is decarboxylated by aromatic-amino-acid decarboxylase to produce serotonin which is converted by arylalkylamine-N-acetyltransferase (AA-NAT) to N-acetylserotonin and finally, through methylation of the hydroxyl group by hydroxyindole O-methyltransferase (HIOMT), converted in N-Acetyl-5-methoxytryptamine (8,9). Melatonin is a potent scavenger of reactive oxygen and nitrogen species (ROS and RNS) (10,11). It also promotes the activity of enzymes which are able to neutralize oxidants (12,13). The melatonin biosynthetic pathway is illustrated in Figure 1 (14).
Physiology
Melatonin binds to two receptor subtypes: MT1 and MT2. These receptors show significant similar molecular characteristics with 55% overall amino acid homology (15). They are G-protein coupled receptors (GPCRs) which both activate and inhibit a constellation of intracellular signaling pathways including downstream gene transcription targets such as extracellular signal-regulated kinases 1/2 (ERK 1/2) and cAMP response element-binding protein (CREB) (16). MT1 and MT2 alter intracellular signaling via alterations in scaffolding proteins, g-protein subtype availability and dimer formation. MT1 and MT2 are primarily found as homodimers but they form heterodimers with both themselves and other GPCRs (17). Moreover, melatonin can act intracellularly binding both cytosolic calmodulin (18,19) and two receptors of the Z-retinoid nuclear receptors family (20).
The secretion of melatonin from the pineal gland is regulated by activation of the β-1-adrenergic receptors (21) which promotes its biosynthesis through AA-NAT expression. Its release is suppressed principally by blue light which is influenced by both light intensity and the duration of exposure (22). Melatonin is released into the systemic circulation achieving plasms concentration between 80 and 120 pg/mL at night and 10–20 pg/mL during the day (23). The distribution of melatonin receptor subtypes is related to precise biologic functions within the complexity of central nervous system signaling (17,24). However, melatonin receptors have been found in peripheral tissues, including heart and arteries, adrenal gland, kidney, lung, liver and in B and T lymphocytes (25). Plasma melatonin redistributes rapidly after its release and is found within mitochondria, entering through oligopeptide transporters PEPT1 and PEPT2, where it acts as an antioxidant (26). There is emerging evidence that melatonin is produced within mitochondria (2,27,28), as evidenced by its lineage to cyanobacteria, the very high mitochondrial concentration (26,29), the results of studies of pinealectomy (26), AANAT localization (30,31) and the observation of its synthesis in mammalian oocytes during maturation (32,33). Melatonin is metabolized by cytochrome P450 enzyme CYP1A2 to 6-hydroxymelatonin, conjugated with sulfuric acid (90%) or glucuronic acid (10%) and finally secreted in the urine. Only 5% of the molecule is excreted unchanged (34).
Beneficial effect of melatonin in sepsis
Melatonin has been demonstrated to improve organ function and to increase survival in several models of sepsis (35-39). The beneficial effects of melatonin in these sepsis models are the result of its action on different pathways, some of which we have summarized in this review. There are few clinical trials, mainly on newborns and pediatric patients, that have shown promising results when melatonin is administered for the treatment of sepsis (40-42).
Antioxidant properties
Sepsis is characterized by an oxidative imbalance with oxidant and antioxidant levels related to illness severity (43-47). Free radicals can lead to the damage of protein, lipids, DNA (48) and affect the function of the glycocalyx (49). Melatonin and its metabolites can scavenge ROS/RNS and their action is referred to as the “free radical scavenging cascade” (50). Melatonin has additive advantages over other antioxidants in preventing oxidative damage (51,52). As melatonin reaches high concentrations within mitochondria (53), and together with its metabolites (11,54,55), it has powerful antioxidant action protecting mitochondria from oxidant injury. Melatonin is also involved in the intra-mitochondrial SIRT3 pathway; SIRT3 is a class 3 histone deacetylase, which protects mitochondria from oxidative stress (56-58). In addition, melatonin stimulates the synthesis of other antioxidant enzymes, including glutathione peroxidase, glutathione reductase, y-glutamyl-cysteine synthetase, glucose-6 phosphate dehydrogenase and catalase (12,13). Experimental sepsis models have demonstrated that melatonin restores glutathione levels (59). Melatonin reduces the levels of malondialdehyde and myeloperoxidase expression in the liver, brain, lung and kidneys and has been demonstrated to reduce hepatic necrosis in septic animals (38). Melanotonin’ s favorable antioxidant properties have been reported in models of cecal ligation and puncture (CLP) induced septic shock and lipopolysaccharide (LPS) induced liver failure (35,39,59).
Anti-inflammatory properties
The initial phase of sepsis is characterized by an exaggerated pro-inflammatory response leading to organ dysfunction and ultimately death. Melatonin has significant anti-inflammatory and anti-apoptotic properties (13,39,60-63) and in several rodent models has been demonstrated to reduce pro-inflammatory cytokines levels (35,38,64). In a rat model of LPS-induced acute lung injury, melatonin attenuated pulmonary inflammation; this was associated with a reduction of nuclear factor kappa-β p65 (NF-κB p65) and tumor necrosis factor-α (TNF-ɑ) expression with an increase of the anti-inflammatory cytokine interleukin 10 (IL-10) (65). Melatonin dose-dependently reduced serum TNF-ɑ and interleukin-6 (IL-6) in a murine model of LPS-induced sepsis (66). Attenuation of the cytokine response was also demonstrated in a murine model of sepsis treated with intraperitoneal melatonin, where melatonin significantly improved the survival rate (39). Several in vitro models have demonstrated that melatonin switched-off NF-κB expression (67,68). In a human umbilical vein endothelial cell (HUVEC-C) model of sepsis, melatonin dose-dependently inhibited NF-κB expression and modulated IL-6 and IL-8 expression (69). These anti-inflammatory effects may be mediated by the modulation of the toll like receptor (TLR) inflammatory cascade (70), the reduction of oxidative stress, NF-κB inhibition or the prevention of apoptosis (71-74).
Prevention of mitochondrial dysfunction
Mitochondria play a key role in sepsis-related redox dysregulation. Sepsis may be characterized by a reversible bioenergetic failure due to mitochondrial dysfunction which leads to impairments in oxygen consumption and hyperlactatemia (75,76). The post-mortem evaluation of septic patients has indicated mitochondrial injury; cardiomyocytes show mitochondrial loss, collapse and vacuoles and renal cells demonstrate hyalinosis and tubular vacuolization (77). Mitochondrial dysfunction may be due to diminished activity of pyruvate decarboxylation due to thiamine deficiency (78-81), phosphorylation and inactivation of pyruvate dehydrogenase, impaired electron transport chain (ETC), microcirculatory shunting (82,83) and nitric oxide (84) and ROS (85) mediated mitochondrial damage. The kidney, heart and brain are those organs with the greatest density of mitochondria and are most susceptible to sepsis-induced mitochondrial dysfunction (86). In a murine model of LPS-induced sepsis, melatonin prevented mitochondrial dysfunction through increasing the ATP:O ratio, augmenting complex IV activity and restoring the respiratory control index (RCI)—the ratio of the rate of mitochondrial oxygen consumption (37). Melatonin further proved to normalize the mitochondrial ATP production in septic mice (87), reversing the inhibitory action of LPS on complexes I and IV and restoring the mitochondrial membrane potential (51,69,88). The capacity of melatonin to reverse mitochondrial damage was further investigated by Zhang et al. in a murine model of sepsis (66). These authors demonstrated that melatonin restored the mitochondrial membrane potential, reduced the levels of endoplasmic reticulum (ER) stress and inhibited the pro-apoptotic activation of caspase 12. In their study inhibition of B-cell receptor associated protein 31 (BAP31) expression, a regulator of ER mediated cell apoptosis, was reestablished by melatonin, probably through the MAPK/ERK pathway (66). Lastly, melatonin protects mitochondria by blocking the overexpression of inducible nitric oxide synthase (iNOS) and the subsequent production of nitric oxide (NO) (89).
Prevention of hepatic injury
Acute hepatic dysfunction is a serious complication of sepsis leading to coagulopathy, dysregulated metabolic homeostasis, altered mental status and death. The beneficial effect of melatonin is well known in chronic liver disease (90-96). Melatonin has hepatoprotective properties though its widely distributed receptors within the liver and observed in melatonin receptor knockout mice (97). Melatonin protects the liver by reducing the production of NO in a model of endotoxemia (98). Its antioxidant properties reduce lipid peroxidation (38,59), malondialdehyde (MDA) levels and increases superoxide dismutase (SOD) in the liver of rats treated with LPS (99). Melatonin can restore the LPS-induced hepatic downregulation of Pregnane X receptor -a regulator of gene transcription- and CYP3A (100), which similarly to CYP450 is reduced by LPS (101,102). A murine model of sepsis-related hepatic failure showed impaired glucose metabolism, increased transaminases, IL-1β, TNF-ɑ and IL-6 and inhibitions of silencing information regulator 1 (SIRT1)—a crucial enzyme involved in cell survival, inflammation and metabolism (103,104)—and signal transducer and activator of transcription 3 (STAT3) (105). When treated with melatonin, septic rats showed improvements in insulin resistance and hepatic gluconeogenesis, reduction of liver enzymes, modulation of inflammatory cytokines, increases in SIRT1 and STAT3 and reduced mortality; effects that were antagonized with EX527 a SIRT1 specific inhibitor (105). Finally, melatonin can prevent hepatocyte apoptosis protecting mice from hepatic failure (106).
Preventing septic cardiomyopathy
Myocardial dysfunction in sepsis is closely tied to worse outcomes (107). Septic cardiomyopathy has been labeled a “junctionopathy” (77), characterized by mitochondrial damage, ER stress, impairment of actin-myosin coupling culminating in reduced ejection fractions, cardiac output and hemodynamic instability (108). Cardiac myocytes express melatonin receptors and melatonin has been tested in several murine models of LPS-related sepsis. When treated with melatonin, myocytes demonstrate improved mitochondrial membrane potential, reduced levels of ER stress and caspase-12 mediated apoptosis (66). Melatonin was further able to restore BAP31 expression, which can prevent mitochondrial DNA damage (109) and apoptosis through the MAPK/ERK pathway. The inhibition of ERK abolished melatonin mediated upregulation of BAP31, indicating a relationship in preserving mitochondrial function (66). Mitochondrial NO synthase, which leads to mitochondrial dysfunction is counteracted by the administration of melatonin (87,88,110-112). LPS induced septic cardiomyopathy in mice is characterized by reduction of SIRT1 expression, increased CK-MB and apoptosis via caspase-3 activation leading to reduced ejection fractions (113). Treatment with melatonin improved cardiac function, lowered CK-MB levels and restored SIRT1 expression, as seen in models of septic hepatic injury (105). Melatonin treated mice displayed increased autophagy, a mechanism that protects cardiomyocytes during stress (114,115) and improves contraction (116). LPS-induced septic cardiomyopathy studies have highlighted receptor-interacting protein kinase 3 (Ripk3) as a potential mediator of the aberrant inflammatory cascade responsible for the sepsis-induced myocardial dysfunction (117). Melatonin appeared to suppress Ripk3 activity, optimizing mitochondrial bioenergetics, modulating ER oxidative stress, and normalizing cardio-protective signaling cascades (including AKT, AMPK, and ERK). Ripk3, when overexpressed, mitigates the cardioprotective action of melatonin (117). In vitro cultures of cardiomyocytes exposed to hypoxia/reoxygenation injury displayed inhibition of p21 activated kinase 2 (Pak2), a known primary mediator for ER stress and ERK involvement (118). Melatonin reversed the inverted the hypoxia/reoxygenation injury and through AMPK-Pak2 axis, inhibited caspase 12 and prevented cell death (118).
Inhibiting nitric oxide production
Sepsis results in the increased expression of inducible cytosolic and mitochondrial isoforms of nitric oxide synthase (mtNOS and iNOS) which increase levels of NO and the subsequent mitochondrial damage (88,119,120). In experimental models of sepsis, melatonin inhibits iNOS and mtNOS isoforms (51,88,98). Inhibition of iNOS lowers NO levels, preventing organ failure and death (98). Furthermore, melatonin can increase the activity of complexes I and IV preventing ROS and RNS production and enhancing the ETC (88,121). In addition, melatonin downregulate NOS activity through calmodulin (18,19,122), which impedes the activation of several calcium-dependent enzymes, such as mtNOS and iNOS (88). N-acetyl-5-methoxy Kynurenamine (AMK), a melatonin metabolite, demonstrated an increased ability in binding calmodulin and reducing nNOS expression (123).
Preventing sepsis related brain dysfunction
Altered mental status is one of the cardinal features of sepsis (124). Sepsis associated encephalopathy has a prevalence of approximately 50% in critically ill patients (125,126). The brain’s high rate of oxygen consumption and relative antioxidant deficiency renders it disproportionately susceptible to oxidative stress (127). ROS damage disrupts the blood-brain-barrier, alters mitochondrial respiration and alters tubulin arrangements (128). Increased ROS production promotes the release of the excitatory neurotransmitter glutamate, which alters gene expression, accelerates the apoptotic cascade, and impairs neuronal viability (129).
Despite its common occurrence, there are no diagnostic tests or biomarkers which aid in the diagnosis and evaluation of this complication (130). Despite the absence of biomarkers of septic encephalopathy a few studies have evaluated the role of melatonin for limiting septic encephalopathy (36,130). In a CLP mouse model of sepsis, administration of melatonin attenuated blood brain barrier dysfunction and cerebral edema via the SIRT-1 pathway (130). Furthermore, administration of melatonin was shown to normalize neurobehavioral dysfunction through expression of brain derived neurotrophic factor glial cell-line derived neurotrophic factor within the hippocampus (36).
Immune enhancing properties
Wu and colleagues demonstrated melatonin modulates the immune response after CLP in rats, reducing IL-1β, diminishing polymorphonuclear infiltration, attenuating oxidative stress and reducing NO levels (35). Melatonin reduces IL-6 production in an LPS model of sepsis (37), switching off inflammasome-dependent cytokine production, preventing mitochondrial dysfunction and inhibiting NF-κB activation (69).
Regulating circadian rhythm
Sleep disturbances and delirium may complicate up to 60% of patients within the intensive care unit (131). Evidence suggests septic patients’ melatonin release is profoundly dysregulated (5) if not completely abolished with loss of the normal diurnal variation (132,133). The precise mechanisms behind these findings remain poorly understood with mRNA expression of circadian master genes Period 2 and cryochrome-1 being reduced in the early stages of sepsis (134). This may affect immune cells which are in part regulated by the circadian rhythm and display diurnal variation in activity (135,136). These cells display time-dependent gene expression which results in altered levels of transcription in a concept termed “circadian-gating” (137,138).
Levels of melatonin in the critically ill
The nocturnal peaks and daytime serum levels have been reported to be severely reduced in critically ill patients with loss of the normal diurnal variation (5,132,139-141).
Pharmacokinetic of melatonin
Oral melatonin is rapidly absorbed from the small intestine by first-order kinetics, with a tmax being achieved after approximately 30–45 minutes (142). The bioavailability of oral melatonin is generally low, ranging from 3% to 33%. The low bioavailability is caused by a considerable first-pass metabolism in the liver. The t1/2 elimination is about 54 minutes. The Cmax and area under the curve (AUC) are highly variable, likely attributable to inter-individual variations in the absorption, distribution, metabolism, and/or excretion of the drug. Patients with cirrhosis demonstrate reduced elimination rates and increased plasma melatonin levels. Healthy volunteers were treated with increasing oral doses of melatonin (20, 30, 50 or 100 mg) without adverse effects (143). Elimination half-life for all doses was 52 minutes. While there is limited pharmacokinetic data in critically ill patients (144,145), a 3 mg oral dose was reported to achieve a peak level at a mean of 16 minutes with a t1/2 elimination of 94 minutes. In this study the maximum serum level observed was 11,040 pg/mL. Pharmacological levels were maintained up to 10 hr following administration and no excessive daytime sleepiness was reported in these patients.
Safety of orally administered melatonin
A review of 195 studies (146) evaluating the effects of melatonin supplementation suggested 11 reported adverse effects amongst study patients. These included subjective worsening of symptoms (asthma, headaches, seizures), transient dizziness and headaches, morning drowsiness and abdominal and back pain. In a meta-analysis of 50 studies evaluating the efficacy of oral melatonin supplementation (1 to 20 mg) (147), nearly half reported adverse effects, often transient, associated with daytime dosing and commonly as drowsiness and fatigue. One g/day of orally-administered melatonin over 30-day (148) noted “drowsiness” as a potential adverse effect, with no statistically significant impact on various clinical parameters (blood pressure, heart rate, ECG, serum chemistry, urine analysis). Three separate studies investigating the use of intravenously-administered melatonin (1.25 mg/kg in healthy, epileptic, and Parkinson’s patients (149), 10 mg/kg in preterm infants and septic neonates (150), and 100 mg in healthy subjects (151) did not report adverse side effects. A double-blind, placebo-controlled study evaluating the utility of 5–20 mg of sublingual melatonin in patients undergoing gynecological surgical procedures likewise didn’t report either dose-dependent or dose-independent symptom (152). The lethal dose 50 (LD 50) of melatonin is reported to be infinity; i.e., it is impossible to administer a large enough dose of melatonin to kill an animal. In summary, melatonin is extremely safe being devoid of clinically significant side effects.
Conclusions
Melatonin is a promising adjunctive therapy for sepsis and with several in vivo studies demonstrating the prevention of organ dysfunction and with improvement in outcomes (35-39). Its beneficial effects potentially derive from its free radical scavenging properties, anti-inflammatory action, plausible role in restoring mitochondrial function and protecting from delirium and brain dysfunction. Several clinical trials exploring the use of melatonin in the pediatric population have shown promising results (40-42,150). The safety profile and minimal side effects of oral melatonin should encourage clinicians to consider using melatonin as adjunctive therapy in patients with severe sepsis and septic shock. The optimal dose of melatonin in the treatment of patients with sepsis is unknown. Currently a clinical trial of antioxidant therapy in patients with septic shock is evaluating a 50 mg nighttime dose of melatonin (NCT03557229). Recently, we have included melatonin at a dose of 6 mg (at 9 pm) in our modified hydrocortisone, ascorbic acid and thiamine protocol (mHAT). Clearly, further clinical research is required to evaluate this safe and exceedingly cheap intervention in the management of sepsis.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Byeon Y, Lee K, Park YI, et al. Molecular cloning and functional analysis of serotonin N-acetyltransferase from the cyanobacterium Synechocystis sp. PCC 6803. J Pineal Res 2013;55:371-6. [PubMed]
- Reiter RJ, Rosales-Corral S, Tan DX, et al. Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol Life Sci 2017;74:3863-81. [Crossref] [PubMed]
- Doherty CJ, Kay SA. Circadian control of global gene expression patterns. Annu Rev Genet 2010;44:419-44. [Crossref] [PubMed]
- Panda S. Circadian physiology of metabolism. Science 2016;354:1008-15. [Crossref] [PubMed]
- Mundigler G, Delle-Karth G, Koreny M, et al. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med 2002;30:536-40. [Crossref] [PubMed]
- Bubenik GA, Konturek SJ. Melatonin and aging: prospects for human treatment. J Physiol Pharmacol 2011;62:13-9. [PubMed]
- Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J 2005;19:176-94. [Crossref] [PubMed]
- Axelrod J, Weissbach H. Enzymatic O-methylation of N-acetylserotonin to melatonin. Science 1960;131:1312. [Crossref] [PubMed]
- Coon SL, Roseboom PH, Baler R, et al. Pineal serotonin N-acetyltransferase: expression cloning and molecular analysis. Science 1995;270:1681-3. [Crossref] [PubMed]
- Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 2011;93:350-84. [Crossref] [PubMed]
- Tan D-X, Manchester LC, Esteban-Zubero E, et al. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015;20:18886-906. [Crossref] [PubMed]
- Pandi-Perumal SR, Trakht I, Srinivasan V, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol 2008;85:335-53. [Crossref] [PubMed]
- Reiter RJ. Interactions of the pineal hormone melatonin with oxygen-centered free radicals: a brief review. Braz J Med Biol Res 1993;26:1141-55. [PubMed]
- Burger MC. ChemDoodle Web Components: HTML5 toolkit for chemical graphics, interfaces, and informatics. J Cheminform 2015;7:35. [Crossref] [PubMed]
- Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol Sci 1996;17:100-2. [Crossref] [PubMed]
- Liu J, Clough SJ, Hutchinson AJ, et al. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu Rev Pharmacol Toxicol 2016;56:361-83. [Crossref] [PubMed]
- Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what's new? Br J Pharmacol 2008;154:1182-95. [Crossref] [PubMed]
- Benítez-King G, Huerto-Delgadillo L, Antón-Tay F. Binding of 3H-melatonin to calmodulin. Life sciences 1993;53:201-7. [Crossref] [PubMed]
- Benítez-King G, Ríos A, Martínez A, Antón-Tay F. In vitro inhibition of Ca2+/calmodulin-dependent kinase II activity by melatonin. Biochim Biophys Acta 1996;1290:191-6. [Crossref] [PubMed]
- Becker-André M, Wiesenberg I, Schaeren-Wiemers N, et al. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J Biol Chem 1994;269:28531-4. [PubMed]
- Nesbitt AD, Leschziner GD, Peatfield RC. Headache, drugs and sleep. Cephalalgia 2014;34:756-66. [Crossref] [PubMed]
- Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 2001;21:6405-12. [Crossref] [PubMed]
- Karasek M, Winczyk K. Melatonin in humans. J Physiol Pharmacol 2006;57 Suppl 5:19-39. [PubMed]
- Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep 2009;61:383-410. [Crossref] [PubMed]
- Ekmekcioglu C. Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother 2006;60:97-108. [Crossref] [PubMed]
- Venegas C, García JA, Escames G, et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res 2012;52:217-27. [Crossref] [PubMed]
- Tan DX, Zheng X, Kong J, et al. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int J Mol Sci 2014;15:15858-90. [Crossref] [PubMed]
- Wang L, Feng C, Zheng X, et al. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J Pineal Res 2017;63:e12429. [Crossref] [PubMed]
- Acuña-Castroviejo D, Escames G, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 2014;71:2997-3025. [Crossref] [PubMed]
- Kerényi NA, Balogh I, Somogyi E, et al. Cytochemical investigation of acetyl-serotonin-transferase activity in the pineal gland. Cell Mol Biol Incl Cyto Enzymol 1979;25:259-62. [PubMed]
- Kerényi NA, Somogyi E, Sótonyi P. Electron microscopic studies on the aryl-sulphatase activity of the pineal gland. Acta Histochem 1975;53:192-7. [PubMed]
- Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 2008;44:280-7. [Crossref] [PubMed]
- Coelho LA, Peres R, Amaral FG, et al. Daily differential expression of melatonin-related genes and clock genes in rat cumulus-oocyte complex: changes after pinealectomy. J Pineal Res 2015;58:490-9. [Crossref] [PubMed]
- Tordjman S, Chokron S, Delorme R, et al. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr Neuropharmacol 2017;15:434-43. [Crossref] [PubMed]
- Wu JY, Tsou MY, Chen TH, et al. Therapeutic effects of melatonin on peritonitis-induced septic shock with multiple organ dysfunction syndrome in rats. J Pineal Res 2008;45:106-16. [Crossref] [PubMed]
- Ji MH, Xia DG, Zhu LY, et al. Short- and Long-Term Protective Effects of Melatonin in a Mouse Model of Sepsis-Associated Encephalopathy. Inflammation 2018;41:515-29. [Crossref] [PubMed]
- Lowes DA, Webster NR, Murphy MP, Galley HF. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth 2013;110:472-80. [Crossref] [PubMed]
- Sener G, Toklu H, Kapucu C, et al. Melatonin protects against oxidative organ injury in a rat model of sepsis. Surg Today 2005;35:52-9. [Crossref] [PubMed]
- Carrillo-Vico A, Lardone PJ, Naji L, et al. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J Pineal Res 2005;39:400-8. [Crossref] [PubMed]
- Gitto E, Karbownik M, Reiter RJ, et al. Effects of melatonin treatment in septic newborns. Pediatr Res 2001;50:756-60. [Crossref] [PubMed]
- El-Gendy FM, El-Hawy MA, Hassan MG. Beneficial effect of melatonin in the treatment of neonatal sepsis. J Matern Fetal Neonatal Med 2018;31:2299-303. [Crossref] [PubMed]
- El Frargy M, El-Sharkawy HM, Attia GF. Use of melatonin as an adjuvant therapy in neonatal sepsis. J Neonatal Perinatal Med 2015;8:227-32. [Crossref] [PubMed]
- Chuang CC, Shiesh SC, Chi CH, et al. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit Care 2006;10:R36. [Crossref] [PubMed]
- Karapetsa M, Pitsika M, Goutzourelas N, et al. Oxidative status in ICU patients with septic shock. Food Chem Toxicol 2013;61:106-11. [Crossref] [PubMed]
- Borrelli E, Roux-Lombard P, Grau GE, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med 1996;24:392-7. [Crossref] [PubMed]
- Carr AC, Rosengrave PC, Bayer S, et al. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care 2017;21:300. [Crossref] [PubMed]
- Evans-Olders R, Eintracht S, Hoffer LJ. Metabolic origin of hypovitaminosis C in acutely hospitalized patients. Nutrition 2010;26:1070-4. [Crossref] [PubMed]
- Takeda K, Shimada Y, Amano M, et al. Plasma lipid peroxides and alpha-tocopherol in critically ill patients. Crit Care Med 1984;12:957-9. [Crossref] [PubMed]
- Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol-Heart Circ Physiol 2006;290:H2247-56. [Crossref] [PubMed]
- Tan DX, Manchester LC, Terron MP, et al. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res 2007;42:28-42. [Crossref] [PubMed]
- Martín M, Macías M, Escames G, et al. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J 2000;14:1677-9. [Crossref] [PubMed]
- Gitto E, Tan DX, Reiter RJ, et al. Individual and synergistic antioxidative actions of melatonin: studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J Pharm Pharmacol 2001;53:1393-401. [Crossref] [PubMed]
- Jou MJ, Peng TI, Reiter RJ, et al. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J Pineal Res 2004;37:55-70. [Crossref] [PubMed]
- Reiter RJ, Tan DX, Mayo JC, et al. Melatonin, longevity and health in the aged: an assessment. Free Radic Res 2002;36:1323-9. [Crossref] [PubMed]
- Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J Pineal Res 2013;54:245-57. [Crossref] [PubMed]
- Chen Y, Qing W, Sun M, et al. Melatonin protects hepatocytes against bile acid-induced mitochondrial oxidative stress via the AMPK-SIRT3-SOD2 pathway. Free Radic Res 2015;49:1275-84. [Crossref] [PubMed]
- Pi H, Xu S, Reiter RJ, et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015;11:1037-51. [Crossref] [PubMed]
- Zhai M, Li B, Duan W, Jing L, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res 2017;63:e12419. [Crossref] [PubMed]
- Sewerynek E, Abe M, Reiter RJ, et al. Melatonin administration prevents lipopolysaccharide-induced oxidative damage in phenobarbital-treated animals. J Cell Biochem 1995;58:436-44. [Crossref] [PubMed]
- Poeggeler B, Saarela S, Reiter RJ, et al. Melatonin--a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann NY Acad Sci 1994;738:419-20. [Crossref] [PubMed]
- Srinivasan V, Mohamed M, Kato H. Melatonin in bacterial and viral infections with focus on sepsis: a review. Recent Pat Endocr Metab Immune Drug Discov 2012;6:30-9. [Crossref] [PubMed]
- Srinivasan V, Pandi-Perumal SR, Spence DW, et al. Melatonin in septic shock: some recent concepts. J Crit Care 2010;25:656.e1-6. [Crossref] [PubMed]
- Zhou L, Zhao D, An H, et al. Melatonin prevents lung injury induced by hepatic ischemia-reperfusion through anti-inflammatory and anti-apoptosis effects. Int Immunopharmacol 2015;29:462-7. [Crossref] [PubMed]
- Yavuz T, Kaya D, Behçet M, et al. Effects of melatonin on Candida sepsis in an experimental rat model. Adv Ther 2007;24:91-100. [Crossref] [PubMed]
- Shang Y, Xu SP, Wu Y, et al. Melatonin reduces acute lung injury in endotoxemic rats. Chin Med J (Engl) 2009;122:1388-93. [PubMed]
- Zhang J, Wang L, Xie W, et al. Melatonin attenuates ER stress and mitochondrial damage in septic cardiomyopathy: A new mechanism involving BAP31 upregulation and MAPK-ERK pathway. J Cell Physiol 2020;235:2847-2856. [Crossref] [PubMed]
- Colombo J, Jardim-Perassi BV, Ferreira JPS, et al. Melatonin Differentially Modulates NF-кB Expression in Breast and Liver Cancer Cells. Anticancer Agents Med Chem 2018;18:1688-94. [Crossref] [PubMed]
- Moniruzzaman M, Ghosal I, Das D, Chakraborty SB. Melatonin ameliorates H(2)O(2)-induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol Res 2018;51:17. [Crossref] [PubMed]
- Lowes DA, Almawash AM, Webster NR, et al. Melatonin and structurally similar compounds have differing effects on inflammation and mitochondrial function in endothelial cells under conditions mimicking sepsis. Br J Anaesth 2011;107:193-201. [Crossref] [PubMed]
- Chuffa LGA, Fioruci-Fontanelli BA, Mendes LO, et al. Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer 2015;15:34. [Crossref] [PubMed]
- da Rosa DP, Forgiarini LF, Silva MB, et al. Antioxidants inhibit the inflammatory and apoptotic processes in an intermittent hypoxia model of sleep apnea. Inflamm Res 2015;64:21-9. [Crossref] [PubMed]
- Li Z, Nickkholgh A, Yi X, et al. Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. J Pineal Res 2009;46:365-72. [Crossref] [PubMed]
- Ozbek E, Ilbey YO, Ozbek M, et al. Melatonin attenuates unilateral ureteral obstruction-induced renal injury by reducing oxidative stress, iNOS, MAPK, and NF-kB expression. J Endourol 2009;23:1165-73. [Crossref] [PubMed]
- Chuang JI, Mohan N, Meltz ML, Reiter RJ. Effect of melatonin on NF-kappa-B DNA-binding activity in the rat spleen. Cell Biol Int 1996;20:687-92. [Crossref] [PubMed]
- Valenza F, Aletti G, Fossali T, et al. Lactate as a marker of energy failure in critically ill patients: hypothesis. Crit Care 2005;9:588-93. [Crossref] [PubMed]
- Vasques F, Duscio E, Romitti F, et al. Septic shock-3 vs 2: an analysis of the ALBIOS study. Crit Care 2018;22:237. [Crossref] [PubMed]
- Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Resp Crit Care Med 2013;187:509-17. [Crossref] [PubMed]
- Donnino MW, Carney E, Cocchi MN, et al. Thiamine deficiency in critically ill patients with sepsis. J Crit Care 2010;25:576-81. [Crossref] [PubMed]
- Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017;151:1229-38. [Crossref] [PubMed]
- Andersen LW, Holmberg MJ, Berg KM, et al. Thiamine as an adjunctive therapy in cardiac surgery: a randomized, double-blind, placebo-controlled, phase II trial. Crit Care 2016;20:92. [Crossref] [PubMed]
- Woolum JA, Abner EL, Kelly A, et al. Effect of Thiamine Administration on Lactate Clearance and Mortality in Patients With Septic Shock. Crit Care Med 2018;46:1747-52. [Crossref] [PubMed]
- De Backer D, Donadello K, Sakr Y, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med 2013;41:791-9. [Crossref] [PubMed]
- Ince C, Mik EG. Microcirculatory and mitochondrial hypoxia in sepsis, shock, and resuscitation. J Appl Physiol 2016;120:226-35. [Crossref] [PubMed]
- Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 2001;1504:46-57. [Crossref] [PubMed]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 2003;552:335-44. [Crossref] [PubMed]
- Veltri KL, Espiritu M, Singh G. Distinct genomic copy number in mitochondria of different mammalian organs. J Cell Physiol 1990;143:160-4. [Crossref] [PubMed]
- López LC, Escames G, Ortiz F, et al. Melatonin restores the mitochondrial production of ATP in septic mice. Neuro Endocrinol Lett 2006;27:623-30. [PubMed]
- Escames G, León J, Macías M, et al. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J 2003;17:932-4. [Crossref] [PubMed]
- Kirkebøen KA, Strand OA. The role of nitric oxide in sepsis--an overview. Acta Anaesthesiol Scand 1999;43:275-88. [Crossref] [PubMed]
- Mortezaee K, Khanlarkhani N. Melatonin application in targeting oxidative-induced liver injuries: A review. J Cell Physiol 2018;233:4015-32. [Crossref] [PubMed]
- Celinski K, Konturek PC, Slomka M, et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease--14 months follow up. J Physiol Pharmacol 2014;65:75-82. [PubMed]
- Gonciarz M, Bielański W, Partyka R, et al. Plasma insulin, leptin, adiponectin, resistin, ghrelin, and melatonin in nonalcoholic steatohepatitis patients treated with melatonin. J Pineal Res 2013;54:154-61. [Crossref] [PubMed]
- Gonciarz M, Gonciarz Z, Bielanski W, et al. The effects of long-term melatonin treatment on plasma liver enzymes levels and plasma concentrations of lipids and melatonin in patients with nonalcoholic steatohepatitis: a pilot study. J Physiol Pharmacol 2012;63:35-40. [PubMed]
- Tahan V, Atug O, Akin H, et al. Melatonin ameliorates methionine- and choline-deficient diet-induced nonalcoholic steatohepatitis in rats. J Pineal Res 2009;46:401-7. [Crossref] [PubMed]
- Crespo I, San-Miguel B, Sánchez DI, et al. Melatonin inhibits the sphingosine kinase 1/sphingosine-1-phosphate signaling pathway in rabbits with fulminant hepatitis of viral origin. J Pineal Res 2016;61:168-76. [Crossref] [PubMed]
- Liang YL, Zhang ZH, Liu XJ, et al. Melatonin protects against apoptosis-inducing factor (AIF)-dependent cell death during acetaminophen-induced acute liver failure. PLoS One 2012;7:e51911. [Crossref] [PubMed]
- Mathes AM. Hepatoprotective actions of melatonin: possible mediation by melatonin receptors. World J Gastroenterol 2010;16:6087-97. [Crossref] [PubMed]
- Crespo E, Macías M, Pozo D, et al. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J 1999;13:1537-46. [Crossref] [PubMed]
- Wang H, Wei W, Shen YX, et al. Protective effect of melatonin against liver injury in mice induced by Bacillus Calmette-Guerin plus lipopolysaccharide. World J Gastroenterol 2004;10:2690-6. [Crossref] [PubMed]
- Xu DX, Wei W, Sun MF, et al. Melatonin attenuates lipopolysaccharide-induced down-regulation of pregnane X receptor and its target gene CYP3A in mouse liver. J Pineal Res 2005;38:27-34. [Crossref] [PubMed]
- Sewer MB, Morgan ET. Nitric oxide-independent suppression of P450 2C11 expression by interleukin-1beta and endotoxin in primary rat hepatocytes. Biochem Pharmacol 1997;54:729-37. [Crossref] [PubMed]
- Monshouwer M, McLellan RA, Delaporte E, et al. Differential effect of pentoxifylline on lipopolysaccharide-induced downregulation of cytochrome P450. Biochem Pharmacol 1996;52:1195-200. [Crossref] [PubMed]
- Vachharajani VT, Liu T, Wang X, et al. Sirtuins Link Inflammation and Metabolism. J Immunol Res 2016;16:8167273. [PubMed]
- Yao H, Tao X, Xu L, et al. Dioscin alleviates non-alcoholic fatty liver disease through adjusting lipid metabolism via SIRT1/AMPK signaling pathway. Pharmacol Res 2018;131:51-60. [Crossref] [PubMed]
- Chen J, Xia H, Zhang L, et al. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed Pharmacother 2019;117:109150. [Crossref] [PubMed]
- Wang H, Xu DX, Lv JW, et al. Melatonin attenuates lipopolysaccharide (LPS)-induced apoptotic liver damage in D-galactosamine-sensitized mice. Toxicology 2007;237:49-57. [Crossref] [PubMed]
- Vieillard-Baron A, Caille V, Charron C, et al. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 2008;36:1701-6. [Crossref] [PubMed]
- Martin L, Derwall M, Al Zoubi S, et al. The Septic Heart: Current Understanding of Molecular Mechanisms and Clinical Implications. Chest 2019;155:427-37. [Crossref] [PubMed]
- Iida R, Ueki M, Yasuda T. Identification of interacting partners of Human Mpv17-like protein with a mitigating effect of mitochondrial dysfunction through mtDNA damage. Free Rad Biol Med 2015;87:336-45. [Crossref] [PubMed]
- Escames G, López LC, Ortiz F, et al. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J 2007;274:2135-47. [Crossref] [PubMed]
- Ortiz F, García JA, Acuña-Castroviejo D, et al. The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: inhibition of iNOS and preservation of nNOS. J Pineal Res 2014;56:71-81. [Crossref] [PubMed]
- Escames G, Acuña-Castroviejo D, López LC, et al. Pharmacological utility of melatonin in the treatment of septic shock: experimental and clinical evidence. J Pharm Pharmacol 2006;58:1153-65. [Crossref] [PubMed]
- Zhang WX, He BM, Wu Y, et al. Melatonin protects against sepsis-induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. Life Sci 2019;217:8-15. [Crossref] [PubMed]
- Doherty J, Baehrecke EH. Life, death and autophagy. Nature Cell Biol 2018;20:1110-7. [Crossref] [PubMed]
- Hsieh CH, Pai PY, Hsueh HW, et al. Complete induction of autophagy is essential for cardioprotection in sepsis. Ann Surg 2011;253:1190-200. [Crossref] [PubMed]
- Ren J, Xu X, Wang Q, Ren SY, et al. Permissive role of AMPK and autophagy in adiponectin deficiency-accentuated myocardial injury and inflammation in endotoxemia. J Mol Cell Cardiol 2016;93:18-31. [Crossref] [PubMed]
- Zhong J, Tan Y, Lu J, et al. Therapeutic contribution of melatonin to the treatment of septic cardiomyopathy: A novel mechanism linking Ripk3-modified mitochondrial performance and endoplasmic reticulum function. Redox Biol 2019;26:101287. [Crossref] [PubMed]
- Wang S, Bian W, Zhen J, et al. Melatonin-Mediated Pak2 Activation Reduces Cardiomyocyte Death Through Suppressing Hypoxia Reoxygenation Injury-Induced Endoplasmic Reticulum Stress. J Cardiovasc Pharmacol 2019;74:20-9. [Crossref] [PubMed]
- Tatoyan A, Giulivi C. Purification and characterization of a nitric-oxide synthase from rat liver mitochondria. J Biol Chem 1998;273:11044-8. [Crossref] [PubMed]
- Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta 1999;1411:437-55. [Crossref] [PubMed]
- Martín M, Macías M, Escames G, et al. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res 2000;28:242-8. [Crossref] [PubMed]
- Dai J, Inscho EW, Yuan L, Hill SM. Modulation of intracellular calcium and calmodulin by melatonin in MCF-7 human breast cancer cells. J Pineal Res 2002;32:112-9. [Crossref] [PubMed]
- Acuña-Castroviejo D, Escames G, López LC, et al. Melatonin and nitric oxide: two required antagonists for mitochondrial homeostasis. Endocrine 2005;27:159-68. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Young GB, Bolton CF, Austin TW, et al. he encephalopathy associated with septic illness. Clin Invest Med 1990;13:297-304. [PubMed]
- Sprung CL, Peduzzi PN, Shatney CH, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med 1990;18:801-6. [Crossref] [PubMed]
- Skaper SD, Floreani M, Ceccon M, et al. Excitotoxicity, oxidative stress, and the neuroprotective potential of melatonin. Ann NY Acad Sci 1999;890:107-18. [Crossref] [PubMed]
- Gupta YK, Gupta M, Kohli K. Neuroprotective role of melatonin in oxidative stress vulnerable brain. Indian J Physiol Pharmacol 2003;47:373-86. [PubMed]
- Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev 2002;54:271-84. [Crossref] [PubMed]
- Zhao L, An R, Yang Y, et al. Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: the role of SIRT1 signaling. J Pineal Res 2015;59:230-9. [Crossref] [PubMed]
- Mistraletti G, Carloni E, Cigada M, et al. Sleep and delirium in the intensive care unit. Minerva Anestesiol 2008;74:329-33. [PubMed]
- Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand 2004;48:679-84. [Crossref] [PubMed]
- Verceles AC, Silhan L, Terrin M, et al. Circadian rhythm disruption in severe sepsis: the effect of ambient light on urinary 6-sulfatoxymelatonin secretion. Intensive Care Med 2012;38:804-10. [Crossref] [PubMed]
- Li CX, Liang DD, Xie GH, et al. Altered melatonin secretion and circadian gene expression with increased proinflammatory cytokine expression in early-stage sepsis patients. Mol Med Rep 2013;7:1117-22. [Crossref] [PubMed]
- Boivin DB, James FO, Wu A, et al. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 2003;102:4143-5. [Crossref] [PubMed]
- Obermann HL, Bauer S. Toll-like receptor 9, what o'clock is it? Immunity 2012;36:159-61. [Crossref] [PubMed]
- Truong KK, Lam MT, Grandner MA, et al. Timing Matters: Circadian Rhythm in Sepsis, Obstructive Lung Disease, Obstructive Sleep Apnea, and Cancer. Ann Am Thorac Soc 2016;13:1144-54. [Crossref] [PubMed]
- Liu J, Malkani G, Shi X, et al. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun 2006;74:4750-6. [Crossref] [PubMed]
- Miyazaki T, Kuwano H, Kato H, et al. Correlation between serum melatonin circadian rhythm and intensive care unit psychosis after thoracic esophagectomy. Surgery 2003;133:662-8. [Crossref] [PubMed]
- Shigeta H, Yasui A, Nimura Y, et al. Postoperative delirium and melatonin levels in elderly patients. Am J Surg 2001;182:449-54. [Crossref] [PubMed]
- Shilo L, Dagan Y, Smorjik Y, et al. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci 1999;317:278-81. [Crossref] [PubMed]
- Andersen LPH, Werner MU, Rosenkilde MM, et al. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol Toxicol 2016;17:8. [Crossref] [PubMed]
- Galley HF, Lowes DA, Allen L, et al. Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res 2014;56:427-38. [Crossref] [PubMed]
- Mistraletti G, Sabbatini G, Taverna M, et al. Pharmacokinetics of orally administered melatonin in critically ill patients. J Pineal Res 2010;48:142-7. [Crossref] [PubMed]
- DeMuro RL, Nafziger AN, Blask DE, et al. The absolute bioavailability of oral melatonin. J Clin Pharmacol 2000;40:781-4. [Crossref] [PubMed]
- Posadzki PP, Bajpai R, Kyaw BM, et al. Melatonin and health: an umbrella review of health outcomes and biological mechanisms of action. BMC Med 2018;16:18. [Crossref] [PubMed]
- Foley HM, Steel AE. Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement Ther Med 2019;42:65-81. [Crossref] [PubMed]
- Nordlund JJ, Lerner AB. The effects of oral melatonin on skin color and on the release of pituitary hormones. J Clin Endocrinol. Metab 1977;45:768-74. [Crossref] [PubMed]
- Antón-Tay F, Díaz JL, Fernández-Guardiola A. On the effect of melatonin upon human brain. Its possible therapeutic implications. Life Sci I 1971;10:841-50. [Crossref] [PubMed]
- Gitto E, Reiter RJ, Cordaro SP, et al. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: beneficial effects of melatonin. Am J Perinatol 2004;21:209-16. [Crossref] [PubMed]
- Andersen LPH, Werner MU, Rosenkilde MM, et al. Pharmacokinetics of high-dose intravenous melatonin in humans. J Clin Pharmacol 2016;56:324-9. [Crossref] [PubMed]
- Naguib M, Samarkandi AH. Premedication with melatonin: a double-blind, placebo-controlled comparison with midazolam. Br J Anaesth 1999;82:875-80. [Crossref] [PubMed]


