Biomarkers of progression of chronic obstructive pulmonary disease (COPD)
Chronic obstructive pulmonary disease (COPD) is a chronic, inflammatory lung disease that arises from exposure to cigarette smoke and other inhaled toxins, and results from a gene-environment interaction (1). Disease progression of COPD is variable, with some patients having a relatively stable course, while others suffer relentless progression leading to severe breathlessness, frequent acute exacerbations of COPD (AECOPD), respiratory failure and death. This review will initially focus on radiological markers, and biological markers (biomarkers) in lung tissue, sputum and blood, which may be useful in predicting disease progression in COPD. Emerging approaches to discovering markers of gene-environment interaction will then be discussed, including exhaled breath analysis, exposure to air pollution, the lung microbiome, and lung ageing.
Measurements of disease progression in patients with COPD
Decline in lung function has been the classical objective measure of progression of COPD over time. However, other clinically important measures have been used in epidemiological studies and clinical trials, including symptoms and health status, exacerbations and health care utilisation, and mortality.
Lung function
Lung function, particularly the forced expiratory volume in 1 second (FEV1), provides an objective, physiological measure of worsening airflow obstruction in COPD. The classic Fletcher and Peto study (2) described variable decline in lung function in a cohort of male workers, with some smokers being more susceptible to accelerated decline. A range of clinical and demographic factors has been shown to influence decline in lung function in COPD, such as environmental and occupational pollutants, cigarette smoking, respiratory infections, exacerbations and comorbidities (3). The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort study of 2,163 patients observed a mean rate of decline in FEV1 of 33 mL/year, with higher rates of FEV1 decline in the presence of current smoking, emphysema and bronchodilator reversibility (4).
Symptoms and exacerbations
The Global Initiative for chronic obstructive lung disease (GOLD) guidelines recommend the inclusion of symptom assessment and exacerbation history, together with measurement of the severity of airflow limitation measured by FEV1 (5). The ECLIPSE study confirmed that patients with 2 or more exacerbations per year (frequent exacerbators) were at higher risk of future exacerbations, and this risk was further increased with more severe airflow limitation (6). Change in health status is also an important measure of disease progression (1).
Mortality
COPD is the third leading cause of death world-wide, after ischaemic heart disease and stroke (7). This high rate of mortality is driven by increased smoking worldwide, reduced mortality from other treatable diseases and an ageing world population (5). Groups at high risk of mortality, as described in the Copenhagen City Heart Study of 10,457 participants, included those with lower baseline FEV1 and excessive longitudinal decline in FEV1, even before the point where their lung function becomes abnormal (8).
It is evident that a multitude of relevant clinical phenotypes portrays the disease progression of COPD, which reflects the heterogeneous and complex nature of this chronic disease.
Biomarkers for disease progression of COPD
Biomarkers are any clinical features, imaging quantification or laboratory-based test markers that characterise disease activity, which are useful for diagnosing and monitoring disease processes and response to therapy. Recent excellent reviews have summarised putative biomarkers for detecting the presence of COPD, characterising COPD phenotypes and monitoring response to treatment (9-11). Biomarkers of acute exacerbations have also been reviewed (12).
Identifying individuals with COPD who are at higher risk of progression would enable more personalised management, in order to slow disease progression. Use of biomarkers would potentially add to existing strategies for smoking avoidance, pharmacotherapy, pulmonary rehabilitation and chronic disease management in COPD. Benefits from measuring biomarkers for COPD progression (and not only susceptibility to COPD) include identifying patients who are rapid decliners in the early stages of the disease, predicting disease progression in all severity groups of COPD, and quantifying response to treatment.
The search for reliable biomarkers in COPD, other than FEV1, is ongoing [e.g., the international efforts by the COPD Biomarker Qualification Consortium (9)]. Providing reliable evidence to validate biomarkers before clinical implementation remains an important challenge. Important issues to be addressed include the accuracy and reliability of biomarkers for the clinical state of interest, evaluation of clinical utility and cost-effectiveness, and real world effectiveness compared to other biomarkers (13). The validation of biomarkers (biomarker qualification) for COPD would be clinically applicable to risk stratification of patients and outcome markers of efficacy and safety in drug development and other clinical trials (9).
Radiological markers for emphysema, airway thickness, bronchiectasis and multi-morbidities
Image biomarkers, especially radiological features of COPD morphology visualised on high resolution computed tomography (CT) chest scans, have been found to be useful predictors of disease progression.
Emphysema and airway wall thickness
High resolution CT is able to assess emphysema and airway disease using quantitative indices (14). Inspiratory vs. expiratory analysis of distribution of parenchymal (emphysema) and functional small airways disease provides information about COPD phenotype (15), and change in lung density over time can itself be measured as an endpoint of COPD progression (16).
Quantitative CT measurements have been associated with outcomes of COPD progression in large cohort studies. Accelerated decline in lung function has been associated with more severe emphysema measured quantitatively by CT (17). The MESA (Multi-Ethnic Study of Atherosclerosis) study found that the presence of centrilobular and panlobular emphysema correlated with increased dyspnoea and reduced exercise capacity (18). Airway wall thickness correlated with reduced lung function and increased symptoms in smokers in a cross-sectional study (19). In the COPDGene study of 1,002 subjects, exacerbations were more frequent in those subjects who had a more severe CT emphysema index, and who displayed increased airway wall thickness (20). A higher CT emphysema index was associated with increased risk of respiratory (21,22) and COPD-specific mortality (23). Airway wall thickness was not independently associated with mortality (22).
Bronchiectasis
Bronchiectasis frequently coexists with COPD. Bronchiectasis is a persistent or progressive condition that is characterised by dilated, thick-walled bronchi that fail to clear airway secretions normally. This leads to bacterial infection and a chronic cough productive of sputum, recurrent infective exacerbations and ultimately, lung destruction and respiratory failure (24). In some COPD patients, bronchiectasis is an incidental finding on CT and may be subclinical, as observed in the ECLIPSE study where the overall prevalence of bronchiectasis was 4% in a highly selected population of milder COPD patients (25). In contrast, studies of moderate to severe COPD have demonstrated a higher prevalence of bronchiectasis of from 30% to 60%, with more extensive bronchiectasis in severe COPD (26-28).
The presence of bronchiectasis influences respiratory infections and other complications of COPD. In a study of patients with moderate to severe COPD, patients with COPD and co-existing bronchiectasis, compared to COPD alone, had more severe airflow obstruction (OR 3.9) and an increased yield of potentially pathogenic microorganisms on sputum culture (OR 3.6) (29). Furthermore, bronchiectasis increased the rate of at least one hospital admission for an AECOPD in the previous year (OR 3.0). In a subsequent study of 201 patients with moderate to severe COPD, the same investigators showed that bronchiectasis was independently associated with increased all-cause mortality (HR 2.5) (30). Conversely, a study of 245 patients with non-cystic fibrosis bronchiectasis in Belgium found that 17% of patients had co-existing COPD (31). Over 5 years of follow-up, patients with both bronchiectasis and COPD had a mortality rate of 55%, which was considerably higher than 13% in patients with bronchiectasis alone (31).
These studies emphasise the clinical impact of coexisting bronchiectasis in patients with COPD, especially in terms of excessive rates of AECOPD and mortality. Detecting bronchiectasis in patients with COPD from their routine HRCT chest scans may therefore be potentially clinically useful, identifying those patients who are predisposed to higher rates of exacerbations and increased mortality.
Coronary artery calcification (CAC)
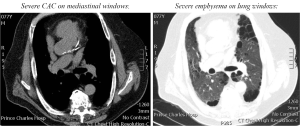
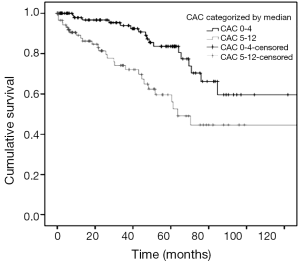
Cardiovascular multi-morbidity is highly prevalent in patients with COPD, and adversely affects mortality. A high prevalence of coronary artery disease has been associated with emphysema severity (32). CAC is a marker of coronary artery disease (Figure 1), and its extent is directly associated with the total burden of coronary atherosclerosis (33). Whilst CAC can be measured using calcium scores on gated, non-contrast CT scans, the use of simple visual scores of CAC has also found utility in lung cancer screening studies (34). In a cross-sectional study of 200 patients with moderate to severe COPD, we observed a high prevalence of CAC (87%) on routine CT chest scans (35). Of prognostic importance, a moderate to high ordinal visual score for CAC (>4 out of a possible 12) was predictive of increased all-cause mortality (HR 2.0) in these patients with COPD (35) (Figure 2). This association was independent of duration of cigarette smoking. Similarly in the ECLIPSE study, a higher coronary artery calcium score percentile was associated with increased mortality (HR 1.77) in COPD patients (36). The results of these radiological studies suggest the scoring of CAC severity on CT chest scans can non-invasively screen for coronary artery disease in patients with COPD with important prognostic implications.
Lung tissue: gene expression markers
Molecular changes in lung parenchyma are a direct reflection of alterations in lung pathology that occur with disease progression in COPD. Routine collection of lung samples is only feasible in patients undergoing lung surgery. Nevertheless, molecular changes in lung tissue provide valuable insight into biomarkers that may be expressed and therefore usefully measured in accessible samples (e.g., sputum, exhaled breath condensate and blood).
A number of studies have used microarrays to examine differences in global mRNA expression between chronic lung disease and normal lung samples (37,38). Other studies have extended this approach by profiling gene expression across different severity stages of COPD. A study of lung tissue from COPD patients (n=21 GOLD stage 0; n=9 stage I; n=10 stage II; n=3 stage III) showed that gene expression correlated with forced expiratory flow between 25% and 75% of forced expiratory volume (FEF25-75%), a measure of small airways function (39). Upregulated genes included those involved in pathways of apoptosis and extracellular matrix synthesis and degradation; down-regulated genes included anti-inflammatory genes. A study of 56 lung tissues (no COPD, to COPD patients from mild to severe) found correlation of FEV1% predicted and FEV1/FVC with functional classes of genes involved in DNA binding and transcription (40).
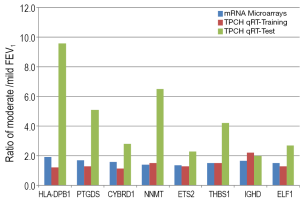
Studies from our group have also provided evidence for differences in gene expression signatures in the earlier stages of COPD disease progression. We have undertaken a study of lung tissue of 30 smokers with emphysema undergoing lung resection for lung cancer, with biological validation in an independent set of 62 patients (41). All patients had airflow limitation with FEV1/VC ratio <0.70 and were arbitrarily classed, based on gas transfer, as mild (KCO ≥75% of predicted) or moderate (KCO <75% of predicted) emphysema. Gene expression profiling and confirmatory PCR identified seven genes that were differentially expressed in moderate emphysema, compared to mild emphysema by more than 1.3-fold: COL6A3, SERPINF1, ZNHIT6, NEDD4, CDKN2A, NRN1 and GSTM3 (41). Our additional study of lung tissue from patients with mild (n=9) or moderate (n=9) COPD, based on FEV1% predicted, with validation in an independent set of 58 lung samples, confirmed differential expression of eight genes (NNMT, THBS1, HLA-DPB1, IGHD, ETS2, ELF1, PTGDS and CYRBD1) by more than 1.8-fold between mild and moderate COPD severity (42) (Figure 3). Ontologies represented by these genes were predominantly cell migration, proliferation, angiogenesis and apoptosis (42). Using the same lung tissue, we have also shown that expression of microRNA-34c is associated with emphysema severity, and modulates SERPINE1 expression in COPD lung (43). Genes and pathways associated with severity of COPD, including the early stages of progression, could therefore be tested as lung biomarkers for progression of emphysema and airflow obstruction in COPD.
Gene expression profiling of specific cells or regions of COPD lung provide additional information about distinct gene signatures for disease progression. Expression of repair genes was examined in 136 paired small airways and emphysema lung tissue obtained by laser capture microdissection from 63 patients (44). Genes involved in tissue destruction were more commonly increased in expression in emphysematous lung tissue and correlated with impaired FEV1, whereas these genes were not as highly expressed in the small airways, thereby promoting bronchiolar remodelling rather than destruction (44). In a study of 238 smokers with or without COPD, gene expression in bronchial brushings was similar to the expression in lung tissue, and this gene expression was regulated in part by activating transcription factor 4 (ATF4) (45). Finally, distinct gene signatures were observed in fibrotic and emphysematous areas of lung, in patients with combined pulmonary fibrosis and emphysema (46). Fibrotic regions expressed genes associated with immune function, and emphysematous areas expressed genes related to cellular fraction, membrane biology, and vascular biology (46), demonstrating that functional differences in gene expression occur with different lung pathologies. Overall, these studies show that specific cells and pathologies in the lung are likely to yield characteristic biomarkers that reflect individual COPD phenotypes of progression.
Sputum: inflammatory cells and mediators
Sputum has been studied as a non-invasive method of sampling biomarkers to assess disease severity and progression in COPD, including exacerbations. Many COPD patients can produce spontaneous sputum samples. However, these often contain a high proportion of non-viable cells which may influence the cell count and mediator profile. To overcome this, sputum can be induced with hypertonic saline in stable patients with COPD, with good safety and reproducibility for cell counts and inflammatory markers (47). Induced sputum also has an adequate safety profile during acute exacerbations, as demonstrated in studies of patients with mild to moderate (48) and moderate to severe COPD (49). Because of many technical and clinical confounding factors (such as interference with assays, smoking status of patients, bacterial infection and concomitant treatment), induced sputum is still undergoing investigation as a source of clinically useful biomarkers (9).
Sputum biomarkers during stability have been associated with severity of COPD. Sputum neutrophil count increased with GOLD stage but was only weakly associated with lung function in the ECLIPSE study (50). Higher levels of human neutrophil peptides (HNP), neutrophil elastase (NE), interleukin (IL)-8 and matrix metalloproteinase (MMP)-9 in spontaneous sputum of COPD patients were associated with greater decline in lung function (FEV1) over 2 years (51). In the ECLIPSE study, microarray profiling of gene expression in induced sputum from 148 COPD patients (and validated in 176 patients) found 277 genes differentially expressed between moderate, severe and very severe GOLD classes, and 198 genes that were differentially expressed between severities of emphysema (52). Further validation is required to test the clinical utility of these genes as biomarkers for COPD progression.
During exacerbations, sputum cell and mediator profiles are heterogeneous and can predict response to therapy of the exacerbation (53). The presence of a mixed inflammatory cell profile in the sputum, together with increased concentrations of sputum and serum biomarkers, were found in patients with exacerbations who had lower FEV1 and increased hospital length of stay (53). Inflammatory mediators in induced sputum during stability may predict future risk of exacerbations. A review by Koutsokera and co-workers found that levels of some mediators in sputum [including in sputum IL-6, IL-8 and myeloperoxidase (MPO)] may be associated with frequency of exacerbations, although more confirmatory studies are needed (12). In a longitudinal study with monthly visits, sputum levels of leukotriene B4 were found to be elevated prior to an exacerbation, and were suggested as possible biomarkers for exacerbation risk (54).
Blood biomarkers: monitoring the systemic compartment
Blood samples provide a convenient source of biomarkers of lung disease. The relevance of blood biomarkers depends on release of markers from the lung into the bloodstream, or systemic markers present in the blood that reflect active disease processes in the lung.
A range of blood biomarkers has been associated with severity of airflow limitation and emphysema. Reduced levels of serum club (Clara) cell protein 16 (CC-16), a protein produced in the lungs and released to the serum, were weakly associated with accelerated decline in lung function (FEV1) in both the Lung Health Study (55) and ECLIPSE study (4). In the TESRA (Treatment of Emphysema with a Selective Retinoid Agonist) and ECLIPSE studies, reduced serum levels of soluble receptor for advanced glycation endproducts (sRAGE) were associated with more severe GOLD stage and more extensive emphysema (56). Lower levels of sRAGE were similarly associated with more advanced emphysema or lower FEV1 in two other studies (57,58). In the ECLIPSE cohort, low levels of vitamin D were correlated with FEV1 and severity of emphysema and associated with 6-minute walk distance, bronchodilator response and CC-16 levels (59). In a subset of the COPDGene cohort, emphysema quantified on CT was associated with higher plasma levels of the adipokine, adiponectin (60) and lower levels of plasma IL-16 (61). Plasma YKL-40 has been associated with higher all-cause mortality (HR 1.4) in a cohort of 493 COPD patients in Denmark (62). Thus a range of biomarkers detectable in peripheral blood show potentially promising relationships with COPD phenotypes.
Panels of blood biomarkers may provide more accurate modelling of future risk. In the Grosshansdorf COPD cohort of 140 COPD patients, clusters of plasma proteins involved in neutrophil function were associated with parameters related to FEV1 (63). Furthermore, proteins related to the epidermal growth factor receptor (EGFR) pathway were associated with gas transfer (DLCO) and FEV1 (63). A panel of three systemic inflammatory markers in peripheral blood (CRP, fibrinogen and leukocyte count) was tested in 6,574 individuals with COPD (defined as FEV1/VC ratio <0.7) in the Copenhagen City Heart Study and the Copenhagen General Population Study (64). Elevation of all three biomarkers simultaneously was associated with an increased risk (OR 3.7) of having frequent exacerbations. This association was observed even in subjects with milder COPD and those with no history of frequent exacerbations (64). In the ECLIPSE study, adding the full range of studied blood biomarkers [white blood cell counts, fibrinogen, chemokine ligand 18, surfactant protein D, CRP, Clara cell secretory protein-16, IL-6, IL-8, tumor necrosis factor α (TNF-α)] to the model of age, BODE index and previous COPD hospitalisations improved prediction of mortality (65). A refined panel of six systemic inflammatory markers in peripheral blood (white cell count, fibrinogen, CRP, IL-6, IL-8, TNF-α) in the ECLIPSE study was able to predict increased mortality and exacerbation rates in COPD patients with inflammation, compared to patients without inflammation (66). Of these, currently plasma fibrinogen is being considered for regulatory qualification as a prognostic marker by the US Food and Drug Administration and the European Medicines Agency (9,67).
Because of the large number of putative biomarkers, heterogeneity in study design and evaluation, a formal systematic review of this emerging field is beyond the scope of this review.
Emerging gene-environment approaches to biomarkers of disease progression in COPD
In addition to the more traditional sampling of biomarkers described above, emerging approaches to capturing the effects of gene-environment interaction on COPD disease progression are receiving more focus in research studies. Of these, analysing biomarkers in exhaled breath is a potentially useful, non-invasive method of sampling the airways and epithelial lining fluid that is exposed to the environment. In addition to cigarette smoking, exposure to air pollution and infection are important environmental drivers of COPD progression and phenotypes. Finally, lung ageing, whilst an endogenous chronological factor, also brings with it exposure to internal and external factors over many years, and should be integrated into the complex profiling of COPD.
Exhaled breath analysis
Volatile organic compounds (VOCs)
Advances in technology have produced small, portable array type devices (electronic noses) that are highly applicable to the clinical setting. Electronic noses use a variety of technologies to emulate the human nose, with VOCs adsorbing onto sensors to produce a change in conductivity, colour or oscillation of a crystal, leading to readouts that are analysed. These devices approach the problem of detection from an entirely different viewpoint from that of the gas chromatograph: in the same way a human nose can tell the difference between the bouquet of chocolate and a rose without needing to know the chemical constituents of the vapour, so the electronic nose is able to discriminate between two vapour mixtures without needing to characterise the exact molecules responsible.
Exhaled breath analysis using differing technologies, including gas chromatography-mass spectrometry and the electronic nose, can discriminate between a range of pulmonary diseases (68), including COPD and asthma (69,70). Relatively few studies to date have linked VOCs profiling of the exhaled breath with COPD progression. A recent study showed that the VOCs pattern is reasonably reproducible in healthy subjects and patients with severe COPD and has some correlation with tests of small airways disease (71). VOCs pattern was shown to differentiate between some phenotypes of COPD, such as patients with higher sputum eosinophilia or frequent exacerbations (72).
Identifying the neutrophilic and eosinophilic inflammatory phenotypes of COPD would further aid in tailoring effective treatment. A strong association between sputum cell count and exhaled breath compounds has been demonstrated in subjects with mild to moderate COPD (GOLD stages I and II) (73). Moreover, VOCs profiling was able to discriminate between subjects with COPD and α1-antitrypsin (AAT) deficiency, with very high accuracy, and the VOCs profile of AAT deficiency patients changed with human recombinant AAT therapy, indicating a possible marker of response to treatment (74). However, before widespread application in the clinical setting, methodological issues of VOCs testing need to be overcome, and more extensive validation is required (75).
Exhaled breath condensate (EBC)
Collection of cooled exhaled breath as condensate is a non-invasive method of sampling the airway lining fluid (76). To date, a small number of studies have examined EBC biomarkers and COPD progression. EBC pH was found to be lower in former smokers with GOLD stage III to IV COPD, compared to stage I (77), suggesting that airway acidification could be a marker of airway inflammation and disease severity in COPD, although not all studies have shown a relationship with FEV1 (78). EBC pH is also reduced during acute exacerbations (79). EBC hydrogen peroxide (H2O2), a marker of oxidative stress, has been shown to correlate with COPD health status as measured by the COPD assessment test (CAT) (80). Methodological issues such as dilution and sensitivity of assays, as well as interpretation of clinical factors that impact on EBC analysis, still require to be solved in larger studies (9).
Exposure to air pollution
The predominant sources of particulate matter in the lungs of COPD patients are cigarette smoke and ambient air pollution (81). With up to 25-45% of patients with chronic airflow limitation being never smokers (82), it is evident that non-smoking-related factors (e.g., air pollution) play a role in the progression of COPD (82,83). Exposure to air pollution should therefore be characterised as a factor that influences disease outcomes in COPD.
Vehicle emissions are a major contributor to air pollution in the urban environment. The main components of vehicle emissions are particulate matter less than 10 μm in diameter (PM10), nitrogen dioxide (NO2) and sulfur dioxide (SO2) (84). Recent epidemiological studies have observed strong associations between air pollution exposure and COPD outcomes, including exacerbations, hospital admissions and mortality (Table 1). The repetitive nature of the inhalation injury caused by air pollution is considered a major mediator in the COPD progression (81). Chronic exposure to air pollution, specifically vehicle emissions, has been linked to increased hospital admissions of COPD patients, including those who are never smokers (89). Analysis of early evidence showed that long-term exposure to particulate matter can lead to a reduction in lung function and increased COPD incidence and progression (81). These studies and others (86,87) support the notion that exposure to air pollution is a driver of COPD progression in susceptible individuals.
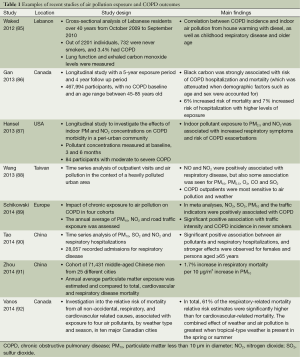
Full table
Monitoring of air quality occurs for legislative and public health requirements, as well as epidemiological research. However, real-time monitoring of personal air pollution exposure and biomarkers of the adverse effects of ambient air pollution are still in development (93). In vitro studies have elucidated gene and protein expression profiles of human bronchial epithelial cells, in response to air pollutant exposure (3), which could be brought to clinical testing with further validation. EBC levels of nitrite and nitrate (markers of oxidative stress) were associated with concentrations of ambient coarse particles, but not indoor air pollutant levels, in four cities in Europe (94). Systemic responses to air pollutants were studied in 242 stable COPD patients in Spain (95). In this time series analysis, blood levels of CRP, fibrinogen, HGF and IL-8 were associated with increased ambient NO2 levels, mainly detected in former smokers.
At present, little is known about the molecular mechanisms by which air pollution can promote progression of COPD, and further studies are needed in this field.
Lung microbiome
Bacteria are strongly associated with AECOPD, with bacteria cultured in ~50% of patients with an AECOPD (96). Chronic airway infection with bacteria (colonisation of the airways by bacteria) is more common in patients with severe COPD (97). Whether chronic infection contributes to the pathogenesis of airway inflammation and increasing frequency and severity of AECOPD is not known. The ‘vicious circle’ hypothesis outlines the principles that chronic microbial colonisation, alters innate immunity and airway epithelial injury contributes to the progression of both COPD and other chronic lung diseases such as bronchiectasis (28,98). According to this paradigm, the presence of chronic bacterial infection in the airways, (including during stable disease), may drive inflammation and disease outcomes.
The microbiome describes the microbial community that share an environment in a particular body site. Next-generation sequencing is used to identify these microbial populations which include microbes that are unculturable (99). Characterising the microbiome is rapidly emerging as an important approach to unravelling the complex microbiology of chronic lung diseases (100) [outlined in detail in this issue of the Journal by Daniel Chambers and colleagues (101)]. The community composition of microbial communities can be determined by sequencing the variable regions of the 16S gene, which encodes bacterial ribosomal RNA (rRNA) (98). Published studies of the lung microbiome in COPD have recruited relatively small numbers of patients, with a range of methods of sampling the microbiome (Table 2). Furthermore, few studies to date have applied study of the lung microbiome to outcomes of COPD progression. In general, tobacco smoking in the absence of COPD does not appear to alter the lung microbiome, but severe COPD is associated with less population diversity of resident bacterial communities, although even this result seems dependent on whether BAL or airway tissue is being sampled (96,103,106).
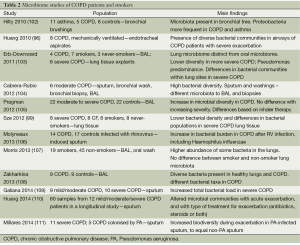
Full table
In COPD, bacterial community profiles in BAL samples from patients using inhaled steroids and long-acting bronchodilators clustered differently from the profiles observed in patients not using these medications (105). Infection with respiratory viruses increases the total bacterial load in patients with COPD, compared to similarly infect healthy controls, but with no obvious difference in bacterial diversity based on analysis of sputum samples (106).
These alterations to the lung microbiome have considerable potential implications for the pathogenesis and progression of COPD. Predominance of one bacterial species in an anatomical lung region (e.g., affected by bronchiectasis) could reduce bacterial diversity, leading to disruption of the balance between mucosal immunity and the bacterial communities present (airway dysbiosis). Alteration of the normal balance of bacterial flora may lead to an excessive inflammatory response, perpetuating the airway inflammation that is characteristic of COPD (98). The microbiome is an emerging source of biomarkers of respiratory infection and possibly COPD progression.
Lung ageing
Ageing is an endogenous rather than exogenous factor, representing cumulative exposures to environmental factors over time. A wide range of phenotypes and biomarkers of ageing are currently being investigated in chronic diseases, including COPD (112,113). Examples of potential relevance to COPD progression include telomere shortening and sirtuins.
Telomeres are protective structures of repetitive sequence that stabilise the ends of chromosome by preserving genetic information and preventing DNA degradation (114,115). Telomere length varies between different cell types, tissues and individuals. Shortening of telomere repeats occurs naturally with cell division, with the shortened telomere ends eventually acting as a signal for apoptosis (116,117). For example, the reduction rate of telomere repeats in peripheral blood mononuclear cells is measured at approximately 84 bp per year, with an accompanying progressive decrease in telomerase activity, in healthy individuals under 40 years of age (118). Telomere length is also a predictor of years of healthy life in older persons (119). Because of this relationship with biological age, telomere length has been associated with ageing and age-related diseases such as COPD. Telomeres are shorter in peripheral blood leukocytes of COPD patients (120,121), particularly cigarette smokers (122,123), providing a common risk factor for accelerated ageing and replicative senescence in COPD.
Telomere length has been linked with lung function in large population studies. A population study of 46,396 subjects (120) found an association between reduced leukocyte telomere length and COPD, and a weak correlation with lung function (FEV1, FVC, FEV1/FVC). A second study found circulating leukocyte telomere length was reduced in patients with COPD (n=934) compared to controls (n=15,846), and more strongly correlated with lung function in never smokers than in smokers (122). Telomeres were found to be relatively preserved in patients with AAT deficiency, compared to non-AAT-related, aged-matched COPD subjects, and there was good correlation between blood and lung telomere lengths (with blood being shorter on average) (124). Of prognostic importance, short leukocyte telomere length was associated with increased risk of all-cause mortality (HR 1.29), compared to longer telomeres, in 4,271 subjects with mild to moderate COPD in the Lung Health Study (125).
Sirtuins (SIRTs) are NAD+-dependent deacetylases and are members of the silent information regulator 2 (Sir2) family (126), with seven homologues in man, SIRT1-7 (127). This family of enzymes is involved in gene silencing and several studies have demonstrated that SIRT1, an anti-inflammatory and anti-ageing protein, is decreased in the lungs of patients with COPD and peripheral blood mononuclear cells in COPD (128-130). Sirtuins also control resistance to oxidative stress and DNA repair (130) and SIRT1 activation reduces cigarette smoke-induced oxidative stress (131). MMP-9 is down regulated by SIRT1 and reduced levels of SIRT1 may cause structural changes in the lung tissue (126,132,133). Sirtuins were shown to be suppressed by cigarette smoking in the large airways of asymptomatic smokers and not in the small airways, whereas in COPD a greater suppression of sirtuin expression was seen in both the large and the small airways (127). Thus there is emerging evidence to suggest that a reduction in sirtuin expression is involved in accelerated lung ageing and pathogenesis of COPD (112).
Conclusions
COPD is a heterogeneous and complex chronic lung disease with extrapulmonary manifestations. Identification of clinically applicable biomarkers would help to screen for and diagnose COPD, monitor disease activity and progression, and guide response to therapy. Similar to other chronic diseases, the search for relevant biomarkers is certainly expanding rapidly in COPD. However, access to samples remains a major issue. Gene expression profiling of lung tissue has identified genes whose expression differs in COPD according to severity, but markers derived from lung tissue are not routinely available for clinical disease monitoring, whereas sputum and blood are readily accessible. Biomarkers in blood, especially inflammatory markers such as fibrinogen, are associated with exacerbations and mortality in larger COPD cohort studies. Much more work is needed to assess blood and sputum biomarkers against disease progression outcomes in COPD. Emerging approaches to studying gene-environment interaction, which impacts on disease pathogenesis and progression in COPD, are providing promising leads for novel biomarkers. These include (I) sampling exhaled breath for VOCs and exhaled breath condensate for protein markers; (II) characterising responses of the lung to inhaled air pollutants; (III) applying knowledge of the lung microbiome to COPD phenotypes; and (IV) determining the significance of biomarkers of ageing such as telomere attrition. Overcoming methodological challenges in sampling and quality control will enable more robust yet easily accessible biomarkers to be developed and applied to optimise personalised medicine in patients with COPD.
Acknowledgements
We thank the patients and staff of The Prince Charles Hospital for their involvement in our research program.
Funding: NHMRC Career Development Fellowship 1026215 (IY), NHMRC Practitioner Fellowship 1019891 (KF), Australian Research Council (ARC) Discovery Grant DP120100126 (IY), Queensland Health and Medical Research project grants (IY, KF, RB), The Prince Charles Hospital Foundation project grants (IY, KF, RB) and PhD scholarship (AV).
Authors’ contributions: All authors contributed to preparing the manuscript, and approved the final version.
Disclosure: The authors declare no conflict of interest.
References
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [PubMed]
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977;1:1645-8. [PubMed]
- Goh F, Shaw JG, Savarimuthu Francis SM, et al. Personalizing and targeting therapy for COPD - the role of molecular and clinical biomarkers. Expert Rev Respir Med 2013;7:593-605. [PubMed]
- Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011;365:1184-92. [PubMed]
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Medical Communications Resources, Inc., 2014. Accessed 12 July 2014. Available online: www.goldcopd.com
- Vestbo J, Agusti A, Wouters EF, et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med 2014;189:1022-30. [PubMed]
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [PubMed]
- Baughman P, Marott JL, Lange P, et al. Combined effect of lung function level and decline increases morbidity and mortality risks. Eur J Epidemiol 2012;27:933-43. [PubMed]
- Casaburi R, Celli B, Crapo J, et al. The COPD Biomarker Qualification Consortium (CBQC). COPD 2013;10:367-77. [PubMed]
- Stockley RA. Biomarkers in chronic obstructive pulmonary disease: confusing or useful? Int J Chron Obstruct Pulmon Dis 2014;9:163-77. [PubMed]
- Agusti A, Sin DD. Biomarkers in COPD. Clin Chest Med 2014;35:131-41. [PubMed]
- Koutsokera A, Kostikas K, Nicod LP, et al. Pulmonary biomarkers in COPD exacerbations: a systematic review. Respir Res 2013;14:111. [PubMed]
- Woodcock J. Assessing the Clinical Utility of Diagnostics Used in Drug Therapy. Clin Pharmacol Ther 2010;88:765-73. [PubMed]
- Litmanovich DE, Hartwick K, Silva M, et al. Multidetector computed tomographic imaging in chronic obstructive pulmonary disease: emphysema and airways assessment. Radiol Clin North Am 2014;52:137-54. [PubMed]
- Galbán CJ, Han MK, Boes JL, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 2012;18:1711-5. [PubMed]
- Coxson HO, Dirksen A, Edwards LD, et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med 2013;1:129-36. [PubMed]
- Mohamed Hoesein FA, de Hoop B, Zanen P, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax 2011;66:782-7. [PubMed]
- Smith BM, Austin JH, Newell JD Jr, et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med 2014;127:94,e7-94,23.
- Dijkstra AE, Postma DS, ten Hacken N, et al. Low-dose CT measurements of airway dimensions and emphysema associated with airflow limitation in heavy smokers: a cross sectional study. Respir Res 2013;14:11. [PubMed]
- Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology 2011;261:274-82. [PubMed]
- Haruna A, Muro S, Nakano Y, et al. CT scan findings of emphysema predict mortality in COPD. Chest 2010;138:635-40. [PubMed]
- Johannessen A, Skorge TD, Bottai M, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med 2013;187:602-8. [PubMed]
- Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema scores predict death from COPD and lung cancer. Chest 2012;141:1216-23. [PubMed]
- Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65 Suppl 1:i1-58. [PubMed]
- Agusti A, Calverley P, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122. [PubMed]
- O’Brien C, Guest PJ, Hill SL, et al. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax 2000;55:635-42. [PubMed]
- Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:400-7. [PubMed]
- Kitch BT, Levy BD, Fanta CH. Late onset asthma: epidemiology, diagnosis and treatment. Drugs Aging 2000;17:385-97. [PubMed]
- Martínez-García MÁ, Soler-Cataluña JJ, Donat Sanz Y, et al. Factors associated with bronchiectasis in patients with COPD. Chest 2011;140:1130-7. [PubMed]
- Martínez-García MA, de la Rosa Carrillo D, Soler-Cataluña JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:823-31. [PubMed]
- Goeminne PC, Nawrot TS, Ruttens D, et al. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med 2014;108:287-96. [PubMed]
- McAllister DA, Maclay JD, Mills NL, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:1208-14. [PubMed]
- Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 1995;92:2157-62. [PubMed]
- Shemesh J, Henschke CI, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology 2010;257:541-8. [PubMed]
- OʼHare PE, Ayres JF. Coronary Artery Calcification on Computed Tomography Correlates With Mortality in Chronic Obstructive Pulmonary Disease. J Comput Assist Tomogr 2014;38:753-9. [PubMed]
- Williams MC, Murchison JT, Edwards LD, et al. Coronary artery calcification is increased in patients with COPD and associated with increased morbidity and mortality. Thorax 2014;69:718-23. [PubMed]
- Yang IA, Francis SM. Deconstructing COPD using genomic tools. Respirology 2009;14:313-7. [PubMed]
- Wheelock CE, Goss VM, Balgoma D, et al. Application of 'omics technologies to biomarker discovery in inflammatory lung diseases. Eur Respir J 2013;42:802-25. [PubMed]
- Wang IM, Stepaniants S, Boie Y, et al. Gene expression profiling in patients with chronic obstructive pulmonary disease and lung cancer. Am J Respir Crit Care Med 2008;177:402-11. [PubMed]
- Bhattacharya S, Srisuma S, Demeo DL, et al. Molecular biomarkers for quantitative and discrete COPD phenotypes. Am J Respir Cell Mol Biol 2009;40:359-67. [PubMed]
- Francis SM, Larsen JE, Pavey SJ, et al. Expression profiling identifies genes involved in emphysema severity. Respir Res 2009;10:81. [PubMed]
- Savarimuthu Francis SM, Larsen JE, Pavey SJ, et al. Genes and Gene Ontologies Common to Airflow Obstruction and Emphysema in the Lungs of Patients with COPD. PLoS ONE 2011;6:e17442. [PubMed]
- Savarimuthu Francis SM, Davidson MR, Tan ME, et al. MicroRNA-34c is associated with emphysema severity and modulates SERPINE1 expression. BMC Genomics 2014;15:88. [PubMed]
- Gosselink JV, Hayashi S, Elliott WM, et al. Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:1329-35. [PubMed]
- Steiling K, van den Berge M, Hijazi K, et al. A dynamic bronchial airway gene expression signature of chronic obstructive pulmonary disease and lung function impairment. Am J Respir Crit Care Med 2013;187:933-42. [PubMed]
- Hanaoka M, Ito M, Droma Y, et al. Comparison of gene expression profiling between lung fibrotic and emphysematous tissues sampled from patients with combined pulmonary fibrosis and emphysema. Fibrogenesis Tissue Repair 2012;5:17. [PubMed]
- Beeh KM, Beier J, Kornmann O, et al. Long-term repeatability of induced sputum cells and inflammatory markers in stable, moderately severe COPD. Chest 2003;123:778-83. [PubMed]
- Bathoorn E, Liesker J, Postma D, et al. Safety of sputum induction during exacerbations of COPD. Chest 2007;131:432-8. [PubMed]
- Gao P, Gibson PG, Zhang J, et al. The safety of sputum induction in adults with acute exacerbation of COPD. Clin Respir J 2013;7:101-9. [PubMed]
- Faner R, Tal-Singer R, Riley JH, et al. Lessons from ECLIPSE: a review of COPD biomarkers. Thorax 2014;69:666-72. [PubMed]
- Paone G, Conti V, Vestri A, et al. Analysis of sputum markers in the evaluation of lung inflammation and functional impairment in symptomatic smokers and COPD patients. Dis Markers 2011;31:91-100. [PubMed]
- Singh D, Fox SM, Tal-Singer R, et al. Induced sputum genes associated with spirometric and radiological disease severity in COPD ex-smokers. Thorax 2011;66:489-95. [PubMed]
- Gao P, Zhang J, He X, et al. Sputum inflammatory cell-based classification of patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One 2013;8:e57678. [PubMed]
- Tufvesson E, Ekberg M, Bjermer L. Inflammatory Biomarkers in Sputum Predict COPD Exacerbations. Lung 2013;191:413-6. [PubMed]
- Park HY, Churg A, Wright JL, et al. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;188:1413-9. [PubMed]
- Cheng DT, Kim DK, Cockayne DA, et al. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;188:948-57. [PubMed]
- Miniati M, Monti S, Basta G, et al. Soluble receptor for advanced glycation end products in COPD: relationship with emphysema and chronic cor pulmonale: a case-control study. Respir Res 2011;12:37. [PubMed]
- Smith DJ, Yerkovich ST, Towers MA, et al. Reduced soluble receptor for advanced glycation end-products in COPD. Eur Respir J 2011;37:516-22. [PubMed]
- Berg I, Hanson C, Sayles H, et al. Vitamin D, vitamin D binding protein, lung function and structure in COPD. Respir Med 2013;107:1578-88. [PubMed]
- Carolan BJ, Kim Y-i, Williams AA, et al. The Association of Adiponectin with Computed Tomography Phenotypes in Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine 2013;188:561-6. [PubMed]
- Bowler RP, Bahr TM, Hughes G, et al. Integrative omics approach identifies interleukin-16 as a biomarker of emphysema. OMICS 2013;17:619-26. [PubMed]
- Holmgaard DB, Mygind LH, Titlestad IL, et al. Plasma YKL-40 and all-cause mortality in patients with chronic obstructive pulmonary disease. BMC Pulm Med 2013;13:77. [PubMed]
- Cockayne DA, Cheng DT, Waschki B, et al. Systemic biomarkers of neutrophilic inflammation, tissue injury and repair in COPD patients with differing levels of disease severity. PLoS One 2012;7:e38629. [PubMed]
- Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013;309:2353-61. [PubMed]
- Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;185:1065-72. [PubMed]
- Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One 2012;7:e37483. [PubMed]
- Duvoix A, Dickens J, Haq I, et al. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax 2013;68:670-6. [PubMed]
- van de Kant KD, van der Sande LJ, Jöbsis Q, et al. Clinical use of exhaled volatile organic compounds in pulmonary diseases: a systematic review. Respir Res 2012;13:117. [PubMed]
- Fens N, Zwinderman AH, van der Schee MP, et al. Exhaled breath profiling enables discrimination of chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med 2009;180:1076-82. [PubMed]
- Fens N, Roldaan AC, van der Schee MP, et al. External validation of exhaled breath profiling using an electronic nose in the discrimination of asthma with fixed airways obstruction and chronic obstructive pulmonary disease. Clin Exp Allergy 2011;41:1371-8. [PubMed]
- Incalzi RA, Pennazza G, Scarlata S, et al. Reproducibility and Respiratory Function Correlates of Exhaled Breath Fingerprint in Chronic Obstructive Pulmonary Disease. PLoS ONE 2012;7:e45396. [PubMed]
- Basanta M, Ibrahim B, Dockry R, et al. Exhaled volatile organic compounds for phenotyping chronic obstructive pulmonary disease: a cross-sectional study. Respir Res 2012;13:72. [PubMed]
- Fens N, de Nijs SB, Peters S, et al. Exhaled air molecular profiling in relation to inflammatory subtype and activity in COPD. Eur Respir J 2011;38:1301-9. [PubMed]
- Hattesohl AD, Jörres RA, Dressel H, et al. Discrimination between COPD patients with and without alpha 1-antitrypsin deficiency using an electronic nose. Respirology 2011;16:1258-64. [PubMed]
- Fens N, van der Schee MP, Brinkman P, et al. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin Exp Allergy 2013;43:705-15. [PubMed]
- Ahmadzai H, Huang S, Hettiarachchi R, et al. Exhaled breath condensate: a comprehensive update. Clin Chem Lab Med 2013;51:1343-61. [PubMed]
- Papaioannou AI, Loukides S, Minas M, et al. Exhaled breath condensate pH as a biomarker of COPD severity in ex-smokers. Respir Res 2011;12:67. [PubMed]
- MacNee W, Rennard SI, Hunt JF, et al. Evaluation of exhaled breath condensate pH as a biomarker for COPD. Respir Med 2011;105:1037-45. [PubMed]
- Warwick G, Thomas PS, Yates DH. Non-invasive biomarkers in exacerbations of obstructive lung disease. Respirology 2013;18:874-84. [PubMed]
- Murata K, Fujimoto K, Kitaguchi Y, et al. Hydrogen peroxide content and pH of expired breath condensate from patients with asthma and COPD. COPD 2014;11:81-7. [PubMed]
- Ling SH, van Eeden SF. Particulate matter air pollution exposure: role in the development and exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2009;4:233-43. [PubMed]
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009;374:733-43. [PubMed]
- Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;182:693-718. [PubMed]
- Ristovski ZD, Miljevic B, Surawski NC, et al. Respiratory health effects of diesel particulate matter. Respirology 2012;17:201-12. [PubMed]
- Waked M, Salame J, Khayat G, Salameh P. Correlates of COPD and chronic bronchitis in nonsmokers: data from a cross-sectional study. Int J Chron Obstruct Pulmon Dis 2012;7:577-85. [PubMed]
- Gan WQ, FitzGerald JM, Carlsten C, et al. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med 2013;187:721-7. [PubMed]
- Hansel NN, McCormack MC, Belli AJ, et al. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:1085-90. [PubMed]
- Wang KY, Chau TT. An Association between Air Pollution and Daily Outpatient Visits for Respiratory Disease in a Heavy Industry Area. PloS one 2013;8:e75220. [PubMed]
- Schikowski T, Adam M, Marcon A, et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur Respir J 2014;44:614-26. [PubMed]
- Tao Y, Mi S, Zhou S, et al. Air pollution and hospital admissions for respiratory diseases in Lanzhou, China. Environmental pollution 2014;185:196-201. [PubMed]
- Zhou M, Liu Y, Wang L, et al. Particulate air pollution and mortality in a cohort of Chinese men. Environ Pollut 2014;186:1-6. [PubMed]
- Vanos JK, Hebbern C, Cakmak S. Risk assessment for cardiovascular and respiratory mortality due to air pollution and synoptic meteorology in 10 Canadian cities. Environ Pollut 2014;185:322-32. [PubMed]
- Kelly FJ, Fuller GW, Walton HA, et al. Monitoring air pollution: Use of early warning systems for public health. Respirology 2012;17:7-19. [PubMed]
- Manney S, Meddings CM, Harrison RM, et al. Association between exhaled breath condensate nitrate + nitrite levels with ambient coarse particle exposure in subjects with airways disease. Occup Environ Med 2012;69:663-9. [PubMed]
- Dadvand P, Nieuwenhuijsen MJ, Agustí À, et al. Air pollution and biomarkers of systemic inflammation and tissue repair in COPD patients. Eur Respir J 2014;44:603-13. [PubMed]
- Huang YJ, Kim E, Cox MJ, et al. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS 2010;14:9-59. [PubMed]
- Rangelov K, Sethi S. Role of infections. Clin Chest Med 2014;35:87-100. [PubMed]
- Sethi S. Chronic obstructive pulmonary disease and infection. Disruption of the microbiome? Ann Am Thorac Soc 2014;11 Suppl 1:S43-7. [PubMed]
- Sze MA, Dimitriu PA, Hayashi S, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;185:1073-80. [PubMed]
- Huang YJ, Charlson ES, Collman RG, et al. The role of the lung microbiome in health and disease. A National Heart, Lung, and Blood Institute workshop report. Am J Respir Crit Care Med 2013;187:1382-7. [PubMed]
- Chambers DC, Gellatly SL, Hugenholtz P, et al. special edition ‘Hot Topics in COPD’—The microbiome in COPD. J Thorac Dis 2014;6:1525-31.
- Hilty M, Burke C, Pedro H, et al. Disordered Microbial Communities in Asthmatic Airways. PLoS ONE 2010;5:e8578. [PubMed]
- Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE 2011;6:e16384. [PubMed]
- Cabrera-Rubio R, Garcia-Nunez M, Seto L, et al. Microbiome diversity in the bronchial tracts of patients with chronic obstructive pulmonary disease. J Clin Microbiol 2012;50:3562-8. [PubMed]
- Pragman AA, Kim HB, Reilly CS, et al. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS ONE 2012;7:e47305. [PubMed]
- Molyneaux PL, Mallia P, Cox MJ, et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;188:1224-31. [PubMed]
- Morris A, Beck JM, Schloss PD, et al. Comparison of the Respiratory Microbiome in Healthy Nonsmokers and Smokers. American Journal of Respiratory and Critical Care Medicine 2013;187:1067-75. [PubMed]
- Zakharkina T, Heinzel E, Koczulla RA, et al. Analysis of the Airway Microbiota of Healthy Individuals and Patients with Chronic Obstructive Pulmonary Disease by T-RFLP and Clone Sequencing. PLoS ONE 2013;8:e68302. [PubMed]
- Galiana A, Aguirre E, Rodriguez JC, et al. Sputum microbiota in moderate versus severe patients with COPD. Eur Respir J 2014;43:1787-90. [PubMed]
- Huang YJ, Sethi S, Murphy T, et al. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol 2014;52:2813-23. [PubMed]
- Millares L, Ferrari R, Gallego M, et al. Bronchial microbiome of severe COPD patients colonised by Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis 2014;33:1101-11. [PubMed]
- MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD). Biochem Soc Trans 2009;37:819-23. [PubMed]
- Lowery EM, Brubaker AL, Kuhlmann E, et al. The aging lung. Clin Interv Aging 2013;8:1489-96. [PubMed]
- Blackburn EH. Structure and function of telomeres. Nature 1991;350:569-73. [PubMed]
- Hemann MT, Strong MA, Hao LY, et al. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 2001;107:67-77. [PubMed]
- Zhang X, Mar V, Zhou W, et al. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev 1999;13:2388-99. [PubMed]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 1961;25:585-621. [PubMed]
- Iwama H, Ohyashiki K, Ohyashiki JH, et al. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum Genet 1998;102:397-402. [PubMed]
- Njajou OT, Hsueh WC, Blackburn EH, et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci 2009;64:860-4. [PubMed]
- Rode L, Bojesen SE, Weischer M, et al. Short telomere length, lung function and chronic obstructive pulmonary disease in 46,396 individuals. Thorax 2013;68:429-35. [PubMed]
- Savale L, Chaouat A, Bastuji-Garin S, et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;179:566-71. [PubMed]
- Albrecht E, Sillanpää E, Karrasch S, et al. Telomere length in circulating leukocytes is associated with lung function and disease. Eur Respir J 2014;43:983-92. [PubMed]
- Babizhayev MA, Savel’yeva EL, Moskvina SN, et al. Telomere length is a biomarker of cumulative oxidative stress, biologic age, and an independent predictor of survival and therapeutic treatment requirement associated with smoking behavior. Am J Ther 2011;18:e209-26. [PubMed]
- Saferali A, Lee J, Sin DD, et al. Longer telomere length in COPD patients with α1-antitrypsin deficiency independent of lung function. PLoS One 2014;9:e95600. [PubMed]
- Lee J, Sandford AJ, Connett JE, et al. The relationship between telomere length and mortality in chronic obstructive pulmonary disease (COPD). PLoS ONE 2012;7:e35567. [PubMed]
- Corbi G, Bianco A, Turchiarelli V, et al. Potential Mechanisms Linking Atherosclerosis and Increased Cardiovascular Risk in COPD: Focus On Sirtuins. Int J Mol Sci 2013;14:12696-713. [PubMed]
- Isajevs S, Strazda G, Kopeika U, et al. Different patterns of lung sirtuin expression in smokers with and without chronic obstructive pulmonary disease. Medicina (Kaunas) 2012;48:552-7. [PubMed]
- Rajendrasozhan S, Yang SR, Kinnula VL, et al. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:861-70. [PubMed]
- Yao H, Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl Pharmacol 2011;254:72-85. [PubMed]
- Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest 2009;135:173-80. [PubMed]
- Yao H, Sundar IK, Ahmad T, et al. SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 2014;306:L816-28. [PubMed]
- Nakamaru Y, Vuppusetty C, Wada H, et al. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J 2009;23:2810-9. [PubMed]
- Brajer B, Batura-Gabryel H, Nowicka A, et al. Concentration of matrix metalloproteinase-9 in serum of patients with chronic obstructive pulmonary disease and a degree of airway obstruction and disease progression. J Physiol Pharmacol 2008;59 Suppl 6:145-52. [PubMed]


