
Effect of operative approach on quality of life following anatomic lung cancer resection
Introduction
Anatomic lung resection and lymphadenectomy remains the mainstay treatment for early non-small-cell lung cancer (NSCLC). While the traditional open thoracotomy approach is still commonly performed, minimally invasive techniques, including video-assisted thoracoscopic surgery (VATS) and more recently robotic-assisted thoracoscopic surgery (RATS), have been increasingly adopted for lobectomy and segmentectomy (1). Multiple studies have compared thoracotomy, VATS, and RATS on the basis of objective perioperative surgical outcomes. Studies have also demonstrated equivalent long-term oncologic outcomes of minimally-invasive approaches (2-4), with additional shorter-term gains in reduced perioperative pain and morbidity (3,5-8), improved pulmonary function (9,10), decreased hospital length of stay and costs (1,3,8,11), and a faster return to normal activities (3,12). Of equal or even greater importance to patients however is their health-related quality of life (QOL) after surgery (13). Patients’ expected postoperative QOL changes during recovery is an important component of preoperative evaluation and plays a critical role for counseling patients and their acceptance of the risks of surgery (14). It is therefore of interest to the literature and clinical practice to understand how surgical approach to pulmonary resection for NSCLC affects patients’ QOL.
Multiple patient-reported outcomes (PRO) assessment tools have been created to measure health-related QOL across a variety of physical, emotional, psychosocial, and cognitive domains. A systematic review by Brunelli et al. [2012] compared PRO on the basis of extent of surgical resection, finding that pneumonectomy had a significant and sustained negative impact on physical and emotional domains (15). However, the impact of surgical approach on QOL following to early-stage NSCLC has been controversial, and the topic has not been reviewed in a systematic fashion. Our aim in the present study is to review the current evidence on the impact of minimally-invasive versus open surgical technique on QOL following lung cancer surgery.
Methods
A systematic review of the literature was performed to identify articles that reported on QOL after anatomic lung cancer surgery comparing thoracoscopic and open surgical approaches. No experimentation on human or animal subjects was included in this review, and ethical approval was therefore not required for its conduct.
Literature search strategy
The literature search was performed using the electronic databases of PubMed and Google Scholar to identify pertinent articles published before January 1, 2019. The following search terms were combined as keywords or MeSH headings: (‘quality of life’ OR ‘patient-reported outcomes’) AND ‘lung cancer’ AND (‘surgery’ OR ‘resection’) AND (‘vats’ or ‘video-assisted’ OR ‘thoracoscopic’ OR ‘robotic’ OR ‘robotic-assisted’ OR ‘rats’). Additional sources were identified in reference sections of retrieved studies. Eligibility was independently assessed by three separate authors (Emily S. Singer, Peter J. Kneuertz, & Jennifer Nishimura). Disagreements among reviewers were resolved by consensus.
Eligibility criteria
Studies were deemed eligible for inclusion if they met the following criteria: (I) study design: clinical trials, retrospective reviews, and prospective cohort studies; (II) participants: human subjects undergoing surgery for lung cancer; (III) intervention: anatomic lung resection by minimally-invasive or open approach; (IV) outcome: patient-reported QOL and symptoms. Studies were excluded based on these criteria: (I) study design: abstracts, case reports, conference presentations, expert opinions, meta-analyses, and systematic reviews; (II) participants: patients undergoing resection for benign disease; (III) intervention: solely non-anatomic resection or pneumonectomy; (IV) outcome: studies that did not report QOL. Additional restrictions included a period of years 1995 to 2018 and articles available in English.
Data extraction and critical appraisal
Data items of interest included study design, publication year, QOL assessment instruments, timing of assessments, number of patients, surgical approach, extent of resection, and QOL outcomes by domains. Included studies were evaluated according to the Downs and Black tool designed for quality assessment of both randomized trials and non-randomized studies. This tool consists of 27 questions and is a validated instrument that assessed reporting, internal and external validity of studies to determine bias (16). We excluded the question on power assessment (#27) in this present review. Studies are considered low quality with scores 0–9, moderate quality with scores 10–18, and high quality with scores of 19 and above.
Results
Quality and quantity of evidence
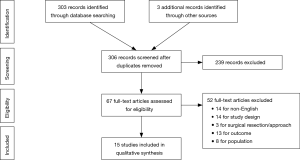
Initial online database query and ancillary search yielded a total of 305 results. After individual review, a total of 15 studies met inclusion criteria. The results of the literature search are summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow chart (Figure 1) (17). Based on the Downs and Black quality scoring system, most of the studies included were of “moderate” quality (11 studies). Four studies, including the only randomized controlled trial, were considered “high” quality. There were no studies of “low” quality.
QOL assessment
In total, eight different tools were employed in the included studies. The most commonly used were the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and the 36-item Short Form health survey (SF-36) (5 studies each), followed by the SF-12 and MDASI (2 studies each). Other instruments, including PROMIS, HADS, FACT-L, 15D, Ferrans and Powers QLI, and the EQ5D were used in only one study each. Five studies employed lung-cancer specific QOL assessment instruments. Separate numeric pain scales were used in four studies. A description of all QOL assessment tools that were used in the included studies is summarized in the Supplement.
Comparison of QOL by operative approach
Table S1 summarizes the characteristics and main findings of these studies. A majority of the studies were observational, and one randomized controlled clinical trial was included. Two studies included robotic-assisted approach in their minimally-invasive group, and one grouped thoracotomy and median sternotomy in the “open” approach. Preoperative or baseline QOL was assessed in 9 studies. Follow-up assessments were conducted postoperatively at different times across studies, with total follow-up period ranging from 4 months to 11 years after surgery.
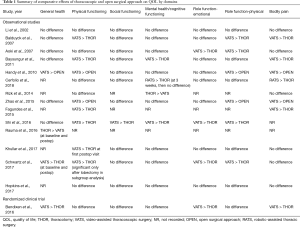
A summary of the QOL domains for each of the eight questionnaires is provided in Table S2. Table 1 further delineates the results of each study based upon seven QOL domains. The results were summarized based upon statistically significant differences in surgical approaches with “>” denoting the surgical approach with a more favorable domain score. A minimally-invasive (VATS or RATS) approach was superior to an open surgical approach in general health (3 studies), physical functioning (9 studies), social functioning (1 study), mental health (3 studies), emotional role functioning (4 studies), physical role functioning (7 studies), and bodily pain (7 studies) (Table 1). The open approach was associated with better general health in one study in which baseline general health among patients undergoing thoracotomy was higher, and was associated with better mental health in another study (Table 1).
Full table
Discussion
PRO have garnered increasing attention in the literature as it now evident that QOL, in addition to traditional surgical outcomes, is of critical importance to patients (18,19). Numerous studies have shown that patients undergoing lung cancer resections may experience significant changes in QOL after lung resection, most severely in the first 6–12 months, but which may last far beyond that (2,15,20-36). The surgical impact on QOL should therefore be part of the early discussion of risks, benefits, and expectations with patients who are candidates for operative resection of early-stage NSCLC. In this review, we found that surgery for lung cancer was well-tolerated regardless of surgical technique, however PRO tended to be better following a minimally-invasive thoracoscopic approach. Importantly, we assessed the effects on the individual QOL domains, and found that physical functioning was most impacted in association with higher pain scores. The majority of the differences in QOL between open and minimally-invasive approaches were seen in the early postoperative period, with more rapid return to baseline self-reported functioning after VATS/RATS compared to thoracotomy.
This review evaluated the spectrum of survey tools, timing, and interpretation of QOL assessment after lung cancer surgery applied in current studies. While eight separate assessment instruments were used among the 15 studies reviewed herein, nearly all addressed issues related to physical symptoms and pain, mental health, and physical, emotional, and social functioning (Table S1). Although, there exist three lung cancer-specific QOL assessment instruments, however only two were used in the studies included in this review with a minority of studies including these instruments (37,38). It is noteworthy that six studies did not include a baseline assessment, suggesting that there is inconsistency in the routine collection of QOL data around the time of lung cancer surgery. These gaps have been attributed to a lack of validated surgical-specific questionnaires as well as the inappropriate consideration of objective parameters as surrogates for QOL outcomes (37). While the evidence presented here suggests that minimally-invasive lung resection leads to better PRO compared to open surgery, there remain opportunities to standardize data collection and integration of PRO into future randomized controlled trials.
The best available piece of evidence on the differential effect of lung cancer surgery by operative approach is derived from the randomized controlled trial reported by Bendixen and colleagues, who compared patients undergoing lobectomy using a 4-port VATS approach versus anterolateral thoracotomy. This trial found that patients in the VATS group reported significantly less clinically relevant moderate to severe pain scores in the immediate recovery period and better self-reported overall QOL during the first 52 weeks after surgery using the EQ5D survey, which incorporates mobility, self-care, usual activity and pain. Interestingly, this trial also used the EORTC QLQ-C30 QOL survey administered at the same time intervals, by which there was no difference in global QOL for the entire study period. However, when analyzed by QOL domains, VATS was associated with higher physical functioning during the first 8 weeks, and better emotional function for the entire study period (36). Similar observations can be made when examining QOL differences between thoracoscopic and open approach in the observational studies included in this study. Although the association of overall QOL by surgical approach varied significantly between studies, physical functioning and pain were the two QOL dimensions which were improved after thoracoscopic lung resection in the majority of studies (Table 1). The effect of operative approach on mental health, emotional and social functioning was less consistent (Table 1).
Several limitations apply to this study, which include the heterogeneity of survey instruments used between studies, the variability of timing of QOL survey administration, which precluded us from performing a meta-analysis. The minimally-invasive approach was VATS in most studies, with a relative scarcity on QOL outcomes after robotic surgery. The present review did not evaluate the impact of extent of resection on QOL as was previously investigated by Brunelli et al. in their 2012 systematic review, which showed that QOL after lung cancer resection is affected by pneumonectomy on a much larger scale. Few studies, in fact, included pneumonectomy patients and may have affected the QOL comparison by approach, as pneumonectomy is most commonly performed via thoracotomy. While tumor size and location tend to dictate the extent of resection, the decision to pursue minimally-invasive versus open surgical approach is more often made at the surgeon’s discretion. Therefore, a thorough understanding of the surgical and PRO that can be expected after each approach should be factored into the practitioner’s operative planning.
Conclusions
QOL assessment after lung cancer surgery was highly variable. In aggregate, current evidence suggests that patients undergoing minimally-invasive pulmonary resection, using a thoracoscopic approach, may result in better QOL during recovery, particularly in the domains of physical functioning and pain as compared with thoracotomy.
Acknowledgments
Funding: This work was supported by the following internal grants. Dr. Kneuertz was supported by The Ohio State University Department of Surgery pilot study award. Dr. D’Souza was supported by The Ohio State University Patient Safety and Advancement grant.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Peter J. Kneuertz) for the series “Patient reported Outcomes in Thoracic Surgery: A new Frontier” published in Journal of Thoracic Disease. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.01.05). The series “Patient reported Outcomes in Thoracic Surgery: A new Frontier” was commissioned by the editorial office without any funding or sponsorship. PJK served as the unpaid Guest Editor of the series. DMD and REM report personal fees from Intuitive Surgical, outside the submitted work. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singer E, Kneuertz PJ, D'Souza DM, et al. Understanding the financial cost of robotic lobectomy: calculating the value of innovation? Ann Cardiothorac Surg 2019;8:194-201. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Cao C, D'Amico T, Demmy T, et al. Less is more: a shift in the surgical approach to non-small-cell lung cancer. Lancet Respir Med 2016;4:e11-2. [Crossref] [PubMed]
- Demmy TL, Yendamuri S. Oncologic validity of minimally invasive lobectomy for early stage lung cancer. J Thorac Dis 2019;11:E163-7. [Crossref] [PubMed]
- Yim AP, Ko KM, Chau WS, et al. Video-assisted thoracoscopic anatomic lung resections. The initial Hong Kong experience. Chest 1996;109:13-7. [Crossref] [PubMed]
- Giudicelli R, Thomas P, Lonjon T, et al. Video-assisted minithoracotomy versus muscle-sparing thoracotomy for performing lobectomy. Ann Thorac Surg 1994;58:712-7; discussion 717-8. [Crossref] [PubMed]
- Kneuertz PJ, D'Souza DM, Moffatt-Bruce SD, et al. Robotic lobectomy has the greatest benefit in patients with marginal pulmonary function. J Cardiothorac Surg 2018;13:56. [Crossref] [PubMed]
- McKenna RJ Jr, Fischel RJ, Wolf R, et al. Video-assisted thoracic surgery (VATS) lobectomy for bronchogenic carcinoma. Semin Thorac Cardiovasc Surg 1998;10:321-5. [Crossref] [PubMed]
- Nakata M, Saeki H, Yokoyama N, et al. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938-41. [Crossref] [PubMed]
- Kaseda S, Aoki T, Hangai N, et al. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg 2000;70:1644-6. [Crossref] [PubMed]
- Nakajima J, Takamoto S, Kohno T, et al. Costs of videothoracoscopic surgery versus open resection for patients with of lung carcinoma. Cancer 2000;89:2497-501. [Crossref] [PubMed]
- Sugiura H, Morikawa T, Kaji M, et al. Long-term benefits for the quality of life after video-assisted thoracoscopic lobectomy in patients with lung cancer. Surg Laparosc Endosc Percutan Tech 1999;9:403-8. [Crossref] [PubMed]
- Kneuertz PJ, Moffatt-Bruce SD. Search for Meaningful Use of Patient-Reported Outcomes in Thoracic Surgery. Ann Thorac Surg 2020;109:1317-8. [Crossref] [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65 Suppl 3:iii1-27. [Crossref] [PubMed]
- Brunelli A, Pompili C, Koller M. Changes in quality of life after pulmonary resection. Thorac Surg Clin 2012;22:471-85. [Crossref] [PubMed]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Cykert S, Kissling G, Hansen CJ. Patient preferences regarding possible outcomes of lung resection: what outcomes should preoperative evaluations target? Chest 2000;117:1551-9. [Crossref] [PubMed]
- Newton-Howes PA, Bedford ND, Dobbs BR, et al. Informed consent: what do patients want to know? N Z Med J 1998;111:340-2. [PubMed]
- Reddy RM. Long-term outcomes and quality of life should be the future focus of research measuring effectiveness of lung cancer surgery approaches. J Thorac Dis 2019;11:361-3. [Crossref] [PubMed]
- Rauma V, Andersson S, Robinson EM, et al. Thoracotomy and VATS surgery in local non-small-cell lung cancer: differences in long-term health-related quality of life. Clin Lung Cancer 2019;20:378-83. [Crossref] [PubMed]
- Avery KNL, Blazeby JM, Chalmers KA, et al. Impact on health-related quality of life of video-assisted thoracoscopic surgery for lung cancer. Ann Surg Oncol 2020;27:1259-71. [Crossref] [PubMed]
- Li WW, Lee TW, Lam SS, et al. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest 2002;122:584-9. [Crossref] [PubMed]
- Khullar OV, Rajaei MH, Force SD, et al. Pilot study to integrate patient reported outcomes after lung cancer operations into the society of thoracic surgeons database. Ann Thorac Surg 2017;104:245-53. [Crossref] [PubMed]
- Schwartz RM, Yip R, Flores RM, et al. The impact of resection method and patient factors on quality of life among stage IA non-small cell lung cancer surgical patients. J Surg Oncol 2017;115:173-80. [Crossref] [PubMed]
- Rauma V, Salo J, Sintonen H, et al. Patient features predicting long-term survival and health-related quality of life after radical surgery for non-small cell lung cancer. Thorac Cancer 2016;7:333-9. [Crossref] [PubMed]
- Zhao J, Zhao Y, Qiu T, et al. Quality of life and survival after II stage nonsmall cell carcinoma surgery: video-assisted thoracic surgery versus thoracotomy lobectomy. Indian J Cancer 2015;52 Suppl 2:e130-3. [Crossref] [PubMed]
- Fagundes CP, Shi Q, Vaporciyan AA, et al. Symptom recovery after thoracic surgery: measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg 2015;150:613-9.e2. [Crossref] [PubMed]
- Rizk NP, Ghanie A, Hsu M, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg 2014;98:1160-6. [Crossref] [PubMed]
- Handy JR Jr, Asaph JW, Douville EC, et al. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg 2010;37:451-5. [PubMed]
- Baysungur V, Tezel Ç, Okur E, et al. Quality of life assessment six months after lobectomy for lung cancer: video-assisted thoracoscopic surgery versus thoracotomy. Turkish Journal of Thoracic and Cardiovascular Surgery 2011;19:207-12. [Crossref]
- Aoki T, Tsuchida M, Hashimoto T, et al. Quality of life after lung cancer surgery: video-assisted thoracic surgery versus thoracotomy. Heart Lung Circ 2007;16:285-9. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life evolution after lung cancer surgery: a prospective study in 100 patients. Lung Cancer 2007;56:423-31. [Crossref] [PubMed]
- Shi Q, Wang XS, Vaporciyan AA, et al. Patient-reported symptom interference as a measure of postsurgery functional recovery in lung cancer. J Pain Symptom Manage 2016;52:822-31. [Crossref] [PubMed]
- Hopkins KG, Ferson PF, Shende MR, et al. Prospective study of quality of life after lung cancer resection. Ann Transl Med 2017;5:204. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Pompili C. Quality of life after lung resection for lung cancer. J Thorac Dis 2015;7:S138-44. [PubMed]
- Hollen PJ, Gralla RJ. Comparison of instruments for measuring quality of life in patients with lung cancer. Semin Oncol 1996;23:31-40. [PubMed]


