
Eighth edition T category is prognostic: the size of the solid component matters, not the ratio
During the past two decades, the prevalence of ground-glass nodule (GGN) and small-sized lung cancer has increased due to the wider use of thin-section computed tomography (CT) and CT screening. Due to its association with smoking, squamous cell carcinoma used to be the most frequent histological type; however, the incidence of adenocarcinoma has recently increased to 60–70% of lung cancer histologic types. This is probably due to the increase in lung cancer patients without smoking history and the higher opportunity for resection of part-solid nodules with ground-glass opacity (GGO). The increasing prevalence of lung adenocarcinoma cases with part-solid nodules enabled many studies to identify the radiological features and oncological characteristics of these tumors. Further, the importance of solid components has been clarified and several studies have attempted to predict the postoperative prognosis using ratios as consolidation-to-tumor ratio (CTR) and tumor disappearance ratio (TDR) (1,2).
The CTR and TDR are closely related to pathological invasion and former studies have shown that evaluating the invasion size after surgical resection contributes to the prediction of the prognosis. In particular, the prognostic impact of CTR has been shown in multiple studies despite differences in the sample sizes and the used methodologies. In previous studies, several cutoff values for CTR (e.g., CTR 0.25 or 0.5) have been proposed to predict the survival of patients who underwent lung resection (1,3-5). On the other hand, Hattori et al. reported that the presence of GGO and the solid component size were independent prognostic factors for overall survival (6).
Based on the prognostic data from the multinational cohort of the International Association for the Study of Lung Cancer (IASLC), small lung cancers (≤3 cm) have been further categorized into T1a (≤1 cm), T1b (>1 to ≤2 cm), and T1c (>2 to ≤3 cm) in the eighth edition of the tumor, node, and metastasis (TNM) classification (7). Further, the eighth edition addressed the correlation between radiologic part-solid nodules and the histologic components of lung adenocarcinomas with a lepidic component and proposed the use of invasive size, rather than the total size, for the T descriptor (8). A validation study that investigated the prognostic impact of invasive size-based staging as compared with that of total size-based staging demonstrated that the use of the invasive size provided better prognostic stratification than the total size (9).
A recent study by Kim et al. (10) investigated the prognostic values of CTR and TDR to clarify whether those prognostic values were independent of the eighth edition clinical T category (cT). The authors hypothesized that there would be a considerable overlap between the prognostic roles of the eighth edition cT and that of CTR and TDR. They conducted a retrospective review of 691 patients with cT1mi to cT1c adenocarcinoma. Multivariate Cox regression analysis showed that age and cT status were independently associated with disease-free survival (DFS), while both CTR and TDR were not independent factors for DFS. This result would be reasonable if we consider a comparison of the two following cases: case 1 with a 3.0 cm tumor and 2.4 cm invasive component (cT1c, CTR 0.8), and case 2 with a 1.0 cm tumor and 0.9 cm invasive component (cT1a, CTR 0.9). One would expect that case 1 will have a poorer prognosis than case 2 although the CTR in case 1 is lower than that in case 2.
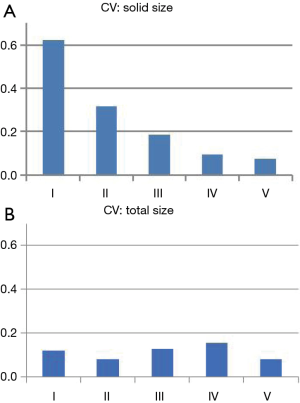
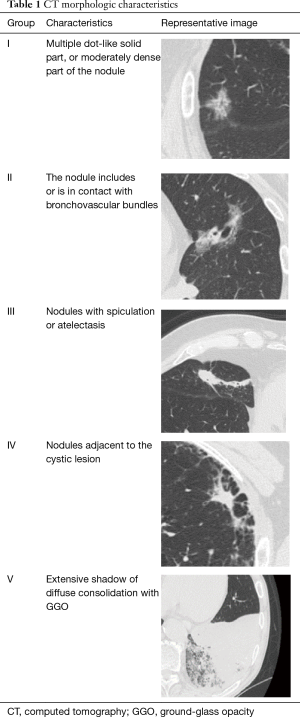
Although this study suggested that measuring the size of the solid component in CT scan would be useful to predict the patient’s prognosis, we should be aware of potential variability in the measurements of the solid component size by preoperative CT. We have demonstrated significant inter-observer variability in size measurement in part-solid adenocarcinomas (11). We assessed the tumor size measurement variability between six physicians (five surgeons and one radiologist). The inter-observer variability in measuring the solid component size was higher than that of measuring the total tumor size in part-solid nodules. Further, small-sized, part-solid adenocarcinoma, which is expected as a minimally-invasive carcinoma showed the highest coefficient value for variation (Figure 1) on the assessment of tumor morphological characteristics in CT patterns (Table 1). These results indicated the difficulty of size measurement of the solid component for part-solid nodules and the existence of unavoidable size measurement variability (11).
Full table
The relationship between pure-solid or part-solid nodules and genetic features, such as EGFR, KRAS, ALK, HER2 (12) and PD-L1 (13) is also being investigated. It is expected that these features will become clearer. Further, it is speculated that the evolution of imaging equipment, such as CT will progress further and that the problem of inter-observer variability may be solved in the future.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.01.50). KS serves as an unpaid editorial board member of Journal of Thoracic Disease from Apr 2019 to Mar 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Haraguchi N, Satoh H, Kikuchi N, et al. Prognostic value of tumor disappearance rate on computed tomography in advanced-stage lung adenocarcinoma. Clin Lung Cancer 2007;8:327-30. [Crossref] [PubMed]
- Matsunaga T, Suzuki K, Takamochi K, et al. What is the radiological definition of part-solid tumour in lung cancer?†. Eur J Cardiothorac Surg 2017;51:242-7. [PubMed]
- Huang TW, Lin KH, Huang HK, et al. The role of the ground-glass opacity ratio in resected lung adenocarcinoma. Eur J Cardiothorac Surg 2018;54:229-34. [Crossref] [PubMed]
- Aokage K, Yoshida J, Ishii G, et al. Identification of early t1b lung adenocarcinoma based on thin-section computed tomography findings. J Thorac Oncol 2013;8:1289-94. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Prognostic impact of a ground glass opacity component in the clinical T classification of non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:2102-10.e1. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1204-23.
- Kameda K, Eguchi T, Lu S, et al. Implications of the eighth edition of the TNM proposal: invasive versus total tumor size for the T descriptor in pathologic stage I-IIA lung adenocarcinoma. J Thorac Oncol 2018;13:1919-29.
- Kim H, Goo JM, Kim YT, et al. Consolidation-to-tumor ratio and tumor disappearance ratio are not independent prognostic factors for the patients with resected lung adenocarcinomas. Lung Cancer 2019;137:123-8. [Crossref] [PubMed]
- Hamanaka K, Takayama H, Koyama T, et al. Interobserver size measurement variability in part-solid lung adenocarcinoma using pre-operative computed tomography. J Thorac Dis 2019;11:2924-31. [Crossref] [PubMed]
- Kobayashi Y, Mitsudomi T, Sakao Y, et al. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann Oncol 2015;26:156-61. [Crossref] [PubMed]
- Suda K, Shimoji M, Shimizu S, et al. Comparison of PD-L1 expression status between pure-solid versus part-solid lung adenocarcinomas. Biomolecules 2019. [Crossref] [PubMed]


