
Long-term outcomes of surgical procedures for Marfan syndrome: aortic dissection versus aneurysm
Introduction
Marfan syndrome (MFS) is an autosomal dominant disorder, which is attributed to mutations in the gene encoding for the extracellular matrix protein fibrillin-1 (1). Aortic root aneurysm and aortic dissection were particularly major concern for these patients though MFS involves multiple organs. Various elective root surgery, including Bentall and valve-sparing root replacement (VSRR), have fostered the concept of prophylactic aortic surgery to prevent type A aortic dissection (TAAD) and its catastrophic sequelae (2). Life expectancy of these patients is therefore prolonged. However, subsequent reinterventions are frequently required on the native thoracic aorta and valves after initial surgery (3).
At first, MFS patients with prior aortic root replacement may at risk of distal dissection since the stiff vascular prosthesis implanted at aortic root may lead to higher pulsatile pressure on the untreated distal aorta (4). It is reported that the risk for distal reintervention in MFS patients are higher in TAAD group than that in aneurysm group, which in favor of the notion that total arch replacement (TAR) combined with frozen elephant trunk (FET) technique should be considered for these patients presenting with TAAD (5). Secondly, for these MFS patients underwent VSRR at initial surgery, reoperation of the preserved aortic valve may be needed during follow-up because of severe aortic regurgitation. In addition, secondary operation may also be required after Bentall procedure due to endocarditis and pseudoaneurysm (6,7). At last, mitral valve prolapse is the second common manifestation of MFS, which may be asymptomatic for a long time. Although the mitral regurgitation is mild or moderate in these MFS patients, secondary mitral valve surgery may be observed during postoperative period since mitral valve dysfunction could progress and develop naturally in MFS patients (3,8).
In brief, despite limited or extended procedures have been performed for MFS patients presenting with aneurysm or dissection, other cardiovascular manifestations were prone to progress inherently. The aim of our study was to determine the full spectrum of subsequent valvular and distal vascular reoperations encountered after initial surgery.
Methods
This study was approved by the Ethics Committee of Changhai Hospital affiliated to Second Military Medical University (project number: 20180630). A total of 201 consecutive patients diagnosed as MFS, which was accordance to Ghent criteria (9), in Changhai Hospital between 2000 to 2019 were enrolled in this study. In brief, limited proximal repair was preferred for MFS patients with aortic root aneurysm, while extended distal repair was the first choice for patients with aortic dissection in our center. As for aortic root repair, Bentall was once the gold standard procedure, while VSRR has been advocated in our center recently. The combination of hemi-arch replacement or TAR and FET was used for TAAD in MFS patients classified into DeBakey type I dissection or DeBakey type II dissection with an enlarged proximal descending aorta or arch >40 mm in diameter. The surgical technique has been depicted in detail previously (10). The final decision for initial procedure was made by surgeon intraoperatively depending on exploration.
Patients’ data were obtained from hospital database and phone contact with patients or their relatives. After discharging at initial surgery, the survivors were followed up in the outpatient clinic at 3 months, 6 months, and 1 year. After that, follow-up was performed when uncomfortable clinical symptoms occurred but at least once per year. Follow-up data included any descriptions of death, reintervention of cardiovascular system, and other adverse events, which consists of endocarditis, hemorrhagic or thromboembolic events. The end-points were defined as all-cause death and reintervention for valve and aorta.
Statistical analysis
All data were analysed retrospectively. Normally distributed continuous variables are depicted as mean ± standard deviation, whereas continuous variables without normal distribution are stated as median (range). Categorical variables are stated as absolute numbers and proportions. Normal distribution of variables was analysed with the Kolmogorov-Smirnov test. Levene’s test was performed for variance homogeneity. Student’s t-tests, Mann-Whitney U test, and Chi-squared or Fisher’s exact test were used appropriately to identify differences between the groups. Kaplan-Meier analysis was used for evaluation of survival and freedom from reinterventions, and the log-rank test was used to test for differences. The Cox regression model was conducted to verify the independent risk factors of distal aortic reoperations. Significant variables associated with subsequent aortic operations in the univariate analysis, including indication was aortic dissection, concomitant CABG, use of β-blocker before initial surgery, weight <65 kg and age <30 years, were included in the multivariate analysis. The statistical analysis was performed by SPSS-V21.0 Software. In all analysis, P<0.05 was considered statistically significant.
Results
Overall outcomes of initial surgery
A total of 201 patients were enrolled. The preoperative profile at initial surgery is presented in Table 1. Mean age of dissection group and aneurysm group were 37.3±11.5 and 36.6±14.3 years, respectively (P=0.687). Forty (37.0%) patients in dissection group and 33 (35.5%) patients in aneurysm group were female (P=0.819). There was no significant difference between both groups concerning patients’ underlying diseases, including hypertension, diabetes mellitus, and coronary atherosclerosis heart disease. Of note, severe aortic regurgitation was in predominating position in both groups that can be observed in 74 (68.5%) patients of dissection group and in 63 (67.7%) patients of aneurysm group. In contrast, majority of patients have normal mitral valve function, which accounts for 87.0% and 76.3% of patients in dissection group and aneurysm group, respectively. The diameters of annulus and ascending aorta in aneurysm group are significantly larger than these diameters in dissection group. It worth mentioning that 3 patients in dissection group and 3 patients in aneurysm group underwent thoracic endovascular aortic repair (TEVAR) procedure previously on account of type B dissection, as well as 1 case underwent prior mitral valve surgery for each group. NUSS procedure was performed in 1 patient in aneurysm group for pectus excavatum repair.
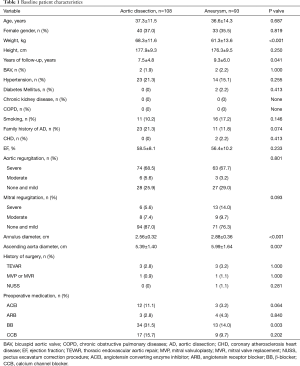
Full table
The operative data are shown in Table 2. The times of cardiopulmonary bypass and cross-clamp in dissection group were 166.1±48.6, 102.3±33.1 minutes, respectively, which are both significantly longer than those times in aneurysm group. In addition, there is no difference between both groups in the time of cerebral perfusion and the hypothermic circulatory arrest temperature. Patients who received Bentall procedure for aortic root repair were in dominant position for dissection group (100 in 108 patients, 92.6%) and aneurysm group (82 in 93 patients, 88.2%). Eighty-three (76.9%) patients in dissection group received distal repair in contrast to 3 (3.2%) patients in aneurysm group. There was no significant difference between both groups regarding concomitant procedures, including tricuspid valvuloplasty and atrial septal defect closure. Seventeen (15.7%) patients in dissection group underwent concomitant coronary artery bypass grafting at initial surgery since coronary artery was teared. Coronary artery bypass grafting was decided to perform for 3 (3.2%) patients in aneurysm group because of right ventricular dysfunction after off pump during initial surgery (P=0.007). In addition, concomitant mitral surgery was necessary in 17 (18.3%) patients presenting with aneurysm and 7 (6.5%) patients with dissection, respectively (P=0.010).
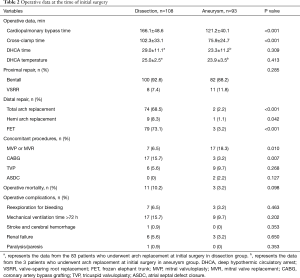
Full table
Overall, the operative mortality rates were 10.2% (11 of 108 patients) in dissection group and 3.2% (3 of 93 patients) in aneurysm group, respectively. The operative mortality rate in dissection group seems to be higher than that in aneurysm group, but without statistical significance (P=0.098). Of the 14 patients who died perioperatively at initial surgery, 9 cases suffered multiorgan failure and sepsis, 3 cases suffered low cardiac output, and another 2 cases suffered distal aortic rupture. The occurrences of complications, which consist of reexploration, prolonged ventilation time, renal failure, paralysis, and cerebral hemorrhage, also seem to be higher in dissection group than those rates in aneurysm group, but without statistical significance. Of note, hemodialysis was required in 9 patients who suffered acute renal failure after initial surgery, 7 of them with poor prognosis. Cerebral hemorrhage was confirmed in 1 case in dissection group perioperatively.
Reinterventions for aortic arch
No patient required secondary arch surgery when the patient underwent TAR at initial surgery. For these patients who failed to receive TAR at initial surgery, secondary TAR+FET became necessary for 11 of 33 patients (33.3%) in dissection group and for 3 of 87 patients (3.4%) in aneurysm group during follow-up. The indication for secondary TAR+FET in 9 patients presenting with dissection was residual dissection of aortic arch and descending aorta. Secondary TAR+FET was performed in another 2 patients before thoracoabdominal replacement since FET can facilitate subsequent surgical procedure. Similarly, 2 cases of secondary TAR+FET for new dissection and 1 case for staged thoracoabdominal replacement were observed in aneurysm group during follow-up.
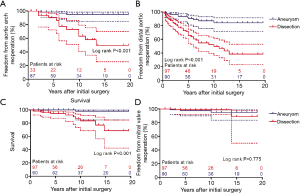
Of these 14 patients received secondary arch procedure, 3 patients died perioperatively. 1 case died of distal aortic rupture, another 2 died of multiorgan failure and sepsis. The in-hospital mortality rate for secondary TAR was 21.4% (3 of 14 patients). For the patients who failed to receive TAR at initial surgery, freedom from aortic arch reoperation in dissection group were 86.2%±6.5%, 63.3%±10.0%, 49.7%±11.8% at 5, 10, and 15 years, respectively. Freedom from aortic arch reoperation in aneurysm group were 98.7%±1.3%, 95.0%±2.9%, 98.7%±1.3% at 5, 10, and 15 years, respectively (Figure 1A, P<0.001).
Reinterventions for distal aorta
Fifty-one patients underwent 73 procedures on distal aortic segments during follow-up, including arch replacement in 11 cases, TEVAR in 53 cases, and thoracoabdominal aortic replacement in 9 cases. Of these 51 patients, 17 patients underwent more than 1 subsequent operation. No patient died perioperatively at secondary operation for descending aortic reintervention. One patient underwent TEVAR died of massive hemorrhage for aortic esophageal fistula 6 years later, and a second patient who received thoracoabdominal aortic replacement died of stent infection 19 months later.
Freedom from distal aortic reoperation in patients with dissection were 65.4%±5.2%, 49.6%±6.4%, and 38.3%±7.7%, and in patients with aneurysm were 90.5%±3.5%, 84.2%±4.8%, and 84.2%±4.8% at 5, 10, and 15 years, respectively (Figure 1B, P<0.001). Table 3 shows the multivariable analysis of risk factors for reoperation. The indication of initial surgery was aortic dissection was demonstrated as the only significant risk factor for subsequent distal operations (P<0.001). In contrast, usage of β-blocker before initial surgery may act as a protective role for subsequent distal operations (P=0.007).
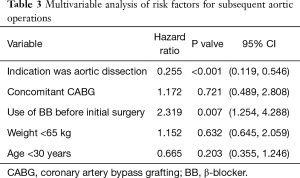
Full table
Survival
During follow-up, cerebral hemorrhage with poor outcome occurred in 1 patient half year after discharging. One patient died of respiratory failure 13 years later. One patient died of cardiac failure. As mentioned before, 3 patients died perioperatively at secondary aortic arch operation, another 2 patients died after reintervention for descending aorta. Another 4 patients died for unknown reason. Survival in patients presenting with dissection were 94.4%±2.4%, 83.4%±5.7%, 68.4%±10.8%, and in patients presenting with aneurysm were 100%, 97.7%±2.3%, 97.7%±2.3% at 5, 10, and 15 years, respectively (Figure 1C, P=0.001).
Reinterventions for mitral valve
Five patients underwent re-sternotomy for mitral valve replacement after initial surgery. Two cases were in dissection group and 3 cases were in aneurysm group. Freedom from mitral valve reoperation in dissection group were 98.8%±1.2%, 98.8%±1.2%, 88.9%±9.4% at 5, 10, and 15 years, respectively. Freedom from mitral valve reoperation in aneurysm group were 97.2%±1.9%, 94.6%±3.2%, 94.6%±3.2% at 5, 10, and 15 years, respectively (Figure 1D, P=0.775).
Discussion
In this paper, we reported the long-term results of our series including 201 MFS patients. Overall, low mortality and satisfactory long-term survival were obtained in both dissection group and aneurysm group by different surgical intervention. Multiple subsequent reinterventions were necessary for these patients during follow-up. In fact, the primary indication for subsequent procedures after initial surgery is the pathological changes in nontreated aortic segments, followed by the changes in mitral valve in our study. Similar results can be observed in Puluca’s research, which enrolled 73 MFS patients (3). Of note, it is reported that the need for subsequent distal procedures is precipitated by an initial presentation with dissection, rather than with aneurysm (5,11,12). Our result also confirmed that the indication of initial surgery was aortic dissection was the only significant independent risk factor for distal aortic reoperations.
The surgical extent at initial surgery may determine the long-term outcome of MFS patients, especially secondary reoperation (13). The surgical strategy of limited proximal repair for aortic root aneurysm in MFS patients have been employed in some cardiac centers, as well as our center (14,15). Notably, concomitant prophylactic arch replacement was considered once the aortic arch was enlarged in aneurysm group in our study.
However, the consensus for extent of initial surgery of MFS patients with TAAD has not been reached. Some group found that limited proximal repair was associated with low mortality in these patients (14). The arch replacement can be performed selectively during follow-up when necessary. While, Bachet et al. observed that subsequent TAR was needed in 14 of 19 patients (73.7%) during follow-up, indicating that the risk for secondary arch operation remains high (15). Furthermore, the in-hospital mortality rate of arch reintervention seems to be higher than that of initial surgery (13). On the other hand, Concistrè et al. observed that patent false lumen may be a risk factor for reoperation (16). Hence, some groups advocated an aggressive distal repair (TAR+TET) at the time of initial surgery for TAAD in MFS patients, which was proved to be superior to limited proximal repair and with lower risk of subsequent reintervention for distal untreated aorta (10). Low perioperative mortality, satisfactory long-term survival, and free from reoperation were reported in Ma’s work by utilizing TAR+TET, which was consistent with our results (10). Collectively, we believe that a more radical approach may be feasible in MFS patients presenting with TAAD when the aortic arch is dissected or enlarged at initial surgery.
The largest proportion of subsequent operation in our cohort was descending aortic procedures, especially in patients with TAAD classified to DeBakey type I at initial surgery. Endovascular repair or open thoracoabdominal aortic replacement were both used for distal repair in our center, especially TEVAR. Endovascular therapy was once treated with caution in MFS patients for its risk of endoleak and surgical conversion (17,18). In our study, TEVAR was performed with low mortality and morbidity rates. It worth mentioning that the rate of reintervention for descending aorta after TEVAR still remain high. The reason could be that the distal aorta continued to dilate despite graft deployment and false lumen thrombosis. Hence, imageological surveillance was imperative for these patients after TEVAR (19).
In addition, 2-staged thoracoabdominal aortic replacement was used for MFS patients failed to received TAR+FET at initial surgery in our center. The advantages of preset FET is that it reduces the complexity in late descending or thoracoabdominal aortic operation by clamping of the elephant trunk without deep hypothermia. Previous studies have reported excellent short-term and long-term effects of 2-staged thoracoabdominal aortic replacement in MFS patients (20). In addition, prior TEVAR for proximal descending thoracic aorta may provide an alternative way for FET before subsequent open thoracoabdominal aortic replacement (21).
As for aortic root repair, Bentall procedure has been verified to be a reliable and durable solution for MFS patients with TAAD regardless of its potential risk of thromboembolic and endocarditis events. VSRR, especially reimplantation technique, has been proved to be a feasible alternative for Bentall by balancing the risk of root reoperation and the benefit of exemption for complication related to mechanical valve (22). Patients received Bentall procedure were in predominant position in our study in both dissection group and aneurysm group. However, VSRR has become the first choice for MFS patients presenting with aneurysm in our center recently. Meanwhile, we have tried to use VSRR technique to repair aortic root in MFS patients with TAAD in recent years. Nevertheless, we still keep a relative conservative attitude toward VSRR when referred to MFS patients with TAAD for our limited experience.
Mitral valve dysfunction would progress gradually without apparent manifestation in MFS patients (23). Mitral prolapse accompanied with mitral regurgitation can be found at the time of initial surgery. Mitral prolapse was present in 82 of 204 MFS patients (40%) in Rybczynski’s research, during which 25 cases developed to severe mitral regurgitation during follow-up (24). The study related to surgical strategy for mitral valve in MFS patients was limited though the prevalence of mitral prolapse was significantly higher than that of general population. Kunkala et al. (8) observed that concomitant mitral procedures at initial surgery did not increase operative risk. In patients with mitral regurgitation grade ≤2 who failed to receive a concomitant mitral procedure, the incidence of progressive mitral regurgitation need subsequent intervention is rare. The similar result was draw in our study, that is concomitant mitral procedures was not necessary when MFS patients with lower mitral regurgitation grades.
Limitation
This investigation was a single-center retrospective study with limited number of subjects. Patients enrolled in our study underwent consecutive surgical interventions during the time period of past 20 years. During that time period, therapeutic strategies have changed which potentially influencing the results.
Conclusions
At initial surgery for MFS patients, limited repair was feasible for aneurysm. However, considering the evidence that TAAD at initial surgery was the only independent predictor for distal aortic reoperation and the high mortality rate of secondary TAR, extended distal repair at initial surgery might be better for MFS patients presenting with TAAD. Concomitant mitral valve procedures may depend on mitral regurgitation grades. The TEVAR and thoracoabdominal aortic replacement were all optional for distal reintervention, but strict postoperative surveillance was necessary.
Acknowledgments
Funding: This study was funded by Shanghai Committee of Science and Technology, China (Grant No. 2014ZYJB0401).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Changhai Hospital affiliated to Second Military Medical University (project number: 20180630).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Judge DP, Dietz HC. Marfan's syndrome. Lancet 2005;366:1965-76. [Crossref] [PubMed]
- Milewicz DM, Dietz HC, Miller DC. Treatment of aortic disease in patients with Marfan syndrome. Circulation 2005;111:e150-7. [Crossref] [PubMed]
- Puluca N, Burri M, Cleuziou J, et al. Consecutive operative procedures in patients with Marfan syndrome up to 28 years after initial aortic root surgery. Eur J Cardiothorac Surg 2018;54:504-9. [Crossref] [PubMed]
- den Hartog AW, Franken R, Zwinderman AH, et al. The risk for type B aortic dissection in Marfan syndrome. J Am Coll Cardiol 2015;65:246-54. [Crossref] [PubMed]
- Girdauskas E, Kuntze T, Borger MA, et al. Distal aortic reinterventions after root surgery in Marfan patients. Ann Thorac Surg 2008;86:1815-9. [Crossref] [PubMed]
- Bernhardt AM, Treede H, Rybczynski M, et al. Comparison of aortic root replacement in patients with Marfan syndrome. Eur J Cardiothorac Surg 2011;40:1052-7. [PubMed]
- Price J, Magruder JT, Young A, et al. Long-term outcomes of aortic root operations for Marfan syndrome: A comparison of Bentall versus aortic valve-sparing procedures. J Thorac Cardiovasc Surg 2016;151:330-6. [Crossref] [PubMed]
- Kunkala MR, Schaff HV, Li Z, et al. Mitral valve disease in patients with Marfan syndrome undergoing aortic root replacement. Circulation 2013;128:S243-7. [Crossref] [PubMed]
- Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476-85. [Crossref] [PubMed]
- Ma WG, Zhang W, Zhu JM, et al. Long-term outcomes of frozen elephant trunk for type A aortic dissection in patients with Marfan syndrome. J Thorac Cardiovasc Surg 2017;154:1175-1189.e2. [Crossref] [PubMed]
- Schoenhoff FS, Jungi S, Czerny M, et al. Acute aortic dissection determines the fate of initially untreated aortic segments in Marfan syndrome. Circulation 2013;127:1569-75. [Crossref] [PubMed]
- Kari FA, Russe MF, Peter P, et al. Late complications and distal growth rates of Marfan aortas after proximal aortic repair. Eur J Cardiothorac Surg 2013;44:163-71. [Crossref] [PubMed]
- Rylski B, Bavaria JE, Beyersdorf F, et al. Type A aortic dissection in Marfan syndrome: extent of initial surgery determines long-term outcome. Circulation 2014;129:1381-6. [Crossref] [PubMed]
- Schoenhoff FS, Kadner A, Czerny M, et al. Should aortic arch replacement be performed during initial surgery for aortic root aneurysm in patients with Marfan syndrome? Eur J Cardiothorac Surg 2013;44:346-51; discussion 351. [Crossref] [PubMed]
- Bachet J, Larrazet F, Goudot B, et al. When should the aortic arch be replaced in Marfan patients? Ann Thorac Surg 2007;83:S774-9; discussion S785-90.
- Concistrè G, Casali G, Santaniello E, et al. Reoperation after surgical correction of acute type A aortic dissection: risk factor analysis. Ann Thorac Surg 2012;93:450-5. [Crossref] [PubMed]
- Marcheix B, Rousseau H, Bongard V, et al. Stent grafting of dissected descending aorta in patients with Marfan's syndrome: mid-term results. JACC Cardiovasc Interv 2008;1:673-80. [Crossref] [PubMed]
- Waterman AL, Feezor RJ, Lee WA, et al. Endovascular treatment of acute and chronic aortic pathology in patients with Marfan syndrome. J Vasc Surg 2012;55:1234-40; discussion 1240-31.
- Nordon IM, Hinchliffe RJ, Holt PJ, et al. Endovascular management of chronic aortic dissection in patients with Marfan syndrome. J Vasc Surg 2009;50:987-91. [Crossref] [PubMed]
- Ikeno Y, Yokawa K, Nakai H, et al. Results of staged repair of aortic disease in patients with Marfan syndrome. J Thorac Cardiovasc Surg 2019;157:2138-2147.e2. [Crossref] [PubMed]
- Johnston WF, Upchurch GR Jr, Tracci MC, et al. Staged hybrid approach using proximal thoracic endovascular aneurysm repair and distal open repair for the treatment of extensive thoracoabdominal aortic aneurysms. J Vasc Surg 2012;56:1495-502. [Crossref] [PubMed]
- Mosbahi S, Stak D, Gravestock I, et al. A systemic review and meta-analysis: Bentall versus David procedure in acute type A aortic dissection. Eur J Cardiothorac Surg 2019;55:201-9. [Crossref] [PubMed]
- van Karnebeek CD, Naeff MS, Mulder BJ, et al. Natural history of cardiovascular manifestations in Marfan syndrome. Arch Dis Child 2001;84:129-37. [Crossref] [PubMed]
- Rybczynski M, Mir TS, Sheikhzadeh S, et al. Frequency and age-related course of mitral valve dysfunction in the Marfan syndrome. Am J Cardiol 2010;106:1048-53. [Crossref] [PubMed]


