In vitro study of coronary flow occlusion in transcatheter aortic valve implantation
Introduction
Surgical aortic valve replacement has been proven to be an effective treatment of symptomatic aortic valve disease with low operative morbidity and mortality in selected patient groups. Traditional surgical approaches require a midline sternotomy and cardiopulmonary bypass with or without cardioplegic arrest. To minimize the invasiveness of operations, a broad spectrum of techniques, including endoscopic and robotic surgery, has been developed and optimized (1-3).
Of note, an increasing number of patients are poor surgical candidates due to advanced age, co-morbidities and previous cardiac surgery. Thus, transcatheter aortic valve implantation (TAVI) was developed and it has been shown to be an effective and feasible procedure in selected patients (4-6), which is believed to benefit a large patient population in the future. Despite the TAVI holds great promise, many technological difficulties and limitations still exist, including potential restriction of coronary ostia, mitral valve insufficiency, and stent migration. Most investigators (7,8) reported the impairment of the coronary blood flow was mainly due to the position of the coronary orifice and the close relationship with the aortic leaflets, valve stent and anterior mitral leaflet during the TAVI. To avoid the impairment of the coronary flow during TAVI, a basic research concerning the relation between valve stent and aortic root anatomic structures including the coronary arterial ostia, aortic leaflets is of paramount importance. Therefore, in current study, we measured the structures of aortic root in 40 fresh adult heart specimens. Through the post mortem aortic valved stent implantation, we investigated the relation of coronary ostia, aortic leaflets, and valved stents, so as to disclose the mechanism of TAVI influence on the coronary blood flow.
Materials and methods
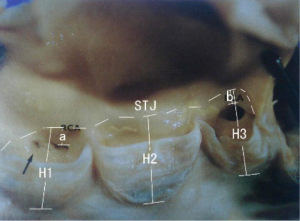
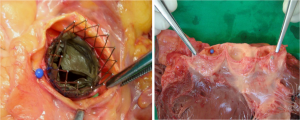
Between March 2007 and August 2007, 40 post mortem hearts were enrolled and dissected in our study, collected at autopsy in the Department of Pathology of University Hospital of Schleswig-Holstein, German. All the cadavers were white adults of both genders. The cadavers were kept in cool place until it was used for autopsy. All the hearts were dissected according to the previously described methods (9). The aorta was opened between the attachment of left and right aortic leaflet. The aortic root including ascending aorta was sectioned sequentially for evaluations as below: first, the ascending aorta was transversally sectioned approximately 1 cm below the arch of aorta and measured the valve size. Second, we removed the aorta at level of the proximal aorta, just approximate 1 cm above the level of sinutubular junction (STJ) of the aorta, and measured the diameter of the proximal aorta, STJ and aortic sinus annulus respectively. Meanwhile, width of aortic leaflet, height of aortic sinus annulus to the STJ level (from the lowest point of aortic sinus annulus to STJ), distance between aortic sinus annulus to its corresponding coronary ostia, and coronary arterial ostia (from the superior margin of the ostia) to its corresponding STJ level were measured (Figure 1). After above measurement was performed, a cut across the aorta above the aortic annulus (approximate 0.5 cm above the STJ) was made to exposure the valve architecture so as to determine the relation of valve stent, aortic leaflets and coronary ostia. Meanwhile, the relationships of valve stent, aortic leaflets and coronary ostia before/post stent implantation and after the open of aorta were evaluated respectively. To illustrate the relation of coronary ostia, aortic leaflets and valve stent, the relation between coronary ostia and leaflets were firstly measured before the stent implantation. Thereafter, we observed the relation among the coronary ostia, leaflets and stents after the stent implantation in the aortic annulus position. Finally, when the aorta was opened, we inspected the aortic leaflets would or not cover the coronary ostia.
Statistical analysis
Descriptive data were reported as either mean ± Standard Deviation, or number and percentage. Continuous variables were compared using Independent Sample T test for normally distributed data. Statistical analysis was performed by using SPSS 16.0 (Chicago, Ill, USA). Statistical significance was defined as a P value <0.05.
Results
There were 19 men and 21 women cadavers were collected in this study. The average age, height, body weight, and heart weight were 69.7 years old, 168.6 cm, 80.6 kg, and 453.1 g respectively.
All 40 hearts possessed three semi-lunar aortic valves and two main coronary ostia. Vast majority coronary arteries originated from the appropriated aortic sinuses, with 34 (85%) left coronary ostia originated from left aortic sinus and 33 (82.5%) right coronary ostia originated from right aortic sinus. The mean diameter of the proximal ascending aorta, STJ, and aortic sinus annulus were 31, 30 and 24 mm respectively. Average size of left and right coronary ostia was 5.3 mm and 4.7 mm respectively.
The mean widths of left, right and posterior aortic leaflet were comparable at 28.92±5.10, 31.22±6.02, and 30.88±5.06 mm respectively. The mean height of left, right and posterior aortic sinus annulus to the related STJ level (from the lowest point of aortic sinus annulus to STJ) was comparable, which was 18.5±2.7, 18.9±2.6, 18.7±2.6 mm, respectively. Likewise, the height of left and right aortic sinus annulus to its corresponding coronary ostia, it was 16.6±2.8 and 17.2±3.1 mm for left and right side respectively. Meanwhile, mean height of left, right and posterior aortic leaflet (from the bottom of aortic valve to the free ridge of the cusp) was 16.1±2.0, 16.4±2.1 and 16.8±1.7 mm respectively. Thus, the height of left and right coronary arterial ostia (from the superior margin of the ostia) to its corresponding STJ level was 1.8±1.1 and 1.8±1.3 mm for two sides respectively.
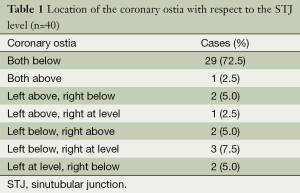
The location of the coronary arterial ostia was measured in relation to the STJ and described as being below, above and at the level of STJ (Table 1). Theoretically, if the height of the aortic leaflet is higher than that of the aortic annulus to its corresponding STJ, this may cause coronary ostia be covered by the aortic leaflet. Of them, both ostia of 17.5% patients were cover by leaflets, while both ostia of 45.0% patients were not covered by leaflets. However, in 12.5% patients, the left ostia was covered while the right ostia was not. Conversely, right ostia of one quarter patients was covered but with left was not (Figure 2). When both ostia lay in the valsalva sinus (below the STJ level), the mean height of the left ostia to STJ line measured 1.9±0.6 mm, and the right was 2.2±0.9 mm. Thus, the right ostia was located lower (deeper) position than the left coronary ostia in the sinus of valsalva.
Full table
Discussion
In this study, we demonstrated that nearly three quarters of the coronary ostia were located below the STJ level and could be covered by the leaflets. This indicated an advanced valve may be necessary to avoid occlusion of coronary blood flow during TAVI.
In certain patients, especially elderly patients with declining overall health status or life-threatening co-morbidities, aortic valve replacement is considered either too risky or contraindicate, because of the significant risk of morbidity and mortality. Furthermore, symptomatic patients with severe aortic stenosis managed medically have a poor prognosis. As an alternative, the transcatheter balloon aortic valvuloplasty is palliative and with an incidence of restenosis, although it may result in temporary improvement of valvular function and relief of symptoms. Given the limited therapeutic options in this subset of patients, TAVI has been an increasingly available therapy for the management of aortic stenosis in high-risk patients (10).
Coronary flow impairment, as a major issue encountered in TAVI, might be caused by the obstruction of coronary ostia by the native leaflets, which was firstly reported by Andersen in 1992. Another in vitro study showed that the deployment of a cylindrical, stented aortic valve can severely affect coronary flow by obstructing the coronary ostia with the native leaflets (11). During orthotopic TAVI, obstruction of coronary ostia, result in coronary flow restriction can occur either by direct blocking from the implanted stent, or from the native aortic leaflets immobilized against the coronary orifices (7,12). Based on this, we observed and investigated the anatomy of aortic root structures, to elucidate the relation of aortic root structures and valved stent, and the influence of valved stents on these anatomic structures by postmortem aortic valve stent implantation.
With respect to the position of coronary ostia in relation to STJ in our study, 29 of 40 (72.5%) hearts analyzed had both ostia located in the aortic sinus, and 11(27.5%) hearts had one or both ostia above or at the STJ level. Of the 80 ostia analyzed in our 40 postmortem hearts, 83.7% (67/80) ostia were located below the sinutubular junctional level, only 7.5% (6/80) ostia were at its level, and 8.8% (7/80) above it. These results are similar to the study by Muriago et al. (13), in which they reported 13% of the ostia at or above that level. On the other hand, our results were different from those of Cavalcanti JS et al. (14) which indicated that 34 of the 50 (68%) hearts studied had one or both ostia above or at the STJ level, while both ostia located below the level only in 32% of cases. Moreover, our study are also different from the study by Waller et al. (15), who have noted up to three-tenths of coronary orifices arising above the STJ. Moreover, the typical vertical and centrically positioning of the ostia in some patients, particularly when both coronary ostia lying above the STJ level, might confer functional advantages for coronary flow during ventricular systole and also prevent the obstruction of the ostia when the aortic valve was opened.
When the valved stents were properly implanted in the postmortem aortic annulus, we found the native aortic leaflets were obviously folded upwards, pushed by valved stents, and immobilized against the leaflets close to the aorta wall. Most of the coronary ostia were partially or fully covered by its native leaflets. In 22 of 40 (55%) of our postmortem study (both covered in seven cases, left in five cases, and right in ten cases), one or both coronary ostia were covered by the aortic leaflets after stents implantation. Among them, 72.4% (21 of 29) cases, which the coronary ostia were located below the STJ, had ostia fully covered by the aortic leaflets. However, only one case (1/11, 9.1%) which the right ostia were located at the STJ level was covered, and the remaining 10 cases which one or both ostia were located at or above the sinutubular level did not have ostia covered. Regarding to the respective ostia, there were 28 of 67 (41.8%) ostia below the STJ were covered, whereas only 1of 13 (7.7%) ostia located at or above the STJ level was covered. Thus, if the coronary ostia lays below the STJ or in the aortic sinus, there is higher frequency they would be covered by their leaflets than ostia located at or above the STJ level. Nevertheless, coronary obstruction after TAVI is rarely observed in the real clinical practice. Coronary obstruction occurred only in 0.72% (3/418) and 0.82% (2/244) of cases after TAVI (16,17). The great discrepancy of incidence of coronary obstruction after TAVI between our study and clinical practice may be because anatomical cover of the coronary ostia shown in our postmortem study is not equal to physiological obstruction of coronary flow on angiography because coronary ostia may be lateral to the stent frame or there may be flow laterally from the open sinuses. Thus, we may overestimate the rate of coronary occlusion in our study. Moreover, different material used in prostheses and their implantation mechanisms may have some influence on the chance of coronary flow impairment. For example, a new-generation SAPIEN 3 valve could prevent the occurrence of this severe complication than earlier generation of balloon-expandable valve (18,19). The cylindrical stent used in out study was prepared by mounting a special material into a self-expandable nitinol stent by means of a suture technique, which was different from more advanced stents currently used for TAVI. A shorter stent placed as low as possible in the aortic annulus has obvious theoretical advantages (11).
Therefore, a good pre-procedural evaluation of the patients in order to avoid this severe complication through measuring the aortic root structure including the height of aortic leaflets, the distance from aortic annulus to its corresponding coronary ostia, or the distance of aortic annulus to STJ by echocardiography (20), CT scan (21) or MRI techniques before the orthotopic TAVI. Moreover, these techniques could accurately assess the calcification of aorta and aortic annulus area (22). Likewise, knowledge of the coronary arteries orifice in relation to the valve plane is critical to prevent inadvertent coronary artery occlusion, and would clearly be beneficial when planning future valve designs (23).
There were several limitations in our study. Firstly, average age of our specimen was 69.7 years old while the reported age for patients underwent TAVI was around 82. Most of the aortic leaflets and annulus tissue of our specimens were normal or mild calcification, which may be different from clinical candidates of TAVI were believed to have severe aortic stenosis and calcification. Secondary, in vitro postmortem aortic valve implantation was performed in our study. It will be more physiological related if we could study in a dynamic and physiological environment in vivo. Lastly, performing such study by using a different style valved stent will be further required. Then, we can compare the options of design a new style valved stent.
In summary, current study demonstrated that most of the coronary ostia were located below the STJ level. This fundamental anatomy may explain potential obstruction of coronary ostia in postmortem orthotopic aortic valved stents implantation. Therefore, new stents should be designed so as to avoid occlusion of coronary blood flow during orthotopic TAVI.
Acknowledgements
This paper was partial supported by Zhejiang Qianjiang Talent Plan (2013R10030) and Zhejiang Province Education Department Project (Y201223135).
Disclosure: The authors declare no conflict of interest.
References
- Rozeik MM, Wheatley DJ, Gourlay T. Percutaneous heart valves; past, present and future. Perfusion 2014;29:397-410. [PubMed]
- Hawkey MC, Lauck SB, Perpetua EM, et al. Transcatheter aortic valve replacement program development: Recommendations for best practice. Catheter Cardiovasc Interv 2014;84:859-67. [PubMed]
- Meredith Am IT, Walters DL, Dumonteil N, et al. Transcatheter aortic valve replacement for severe symptomatic aortic stenosis using a repositionable valve system: 30-day primary endpoint results from the REPRISE II study. J Am Coll Cardiol 2014;64:1339-48. [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [PubMed]
- Varela-Lema L, Queiro-Verdes T, Baz-Alonso JA, et al. Post-introduction observation of transcatheter aortic valve implantation in Galicia (Spain). J Eval Clin Pract 2014. [Epub ahead of print]. [PubMed]
- Ben-Dor I, Goldstein SA, Waksman R, et al. Effects of percutaneous aortic valve replacement on coronary blood flow assessed with transesophageal Doppler echocardiography in patients with severe aortic stenosis. Am J Cardiol 2009;104:850-5. [PubMed]
- Bourantas CV, Serruys PW. Evolution of transcatheter aortic valve replacement. Circ Res 2014;114:1037-51. [PubMed]
- Chapman CB. On the study of the heart: a comment on autopsy techniques. Arch Intern Med 1964;113:318-22. [PubMed]
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004;43:698-703. [PubMed]
- Flecher EM, Curry JW, Joudinaud TM, et al. Coronary flow obstruction in percutaneous aortic valve replacement. An in vitro study. Eur J Cardiothorac Surg 2007;32:291-4; discussion 295. [PubMed]
- Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement: developed in collabration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Thorac Cardiovasc Surg 2012;144:e29-84. [PubMed]
- Muriago M, Sheppard MN, Ho SY, et al. Location of the coronary arterial orifices in the normal heart. Clin Anat 1997;10:297-302. [PubMed]
- Cavalcanti JS, de Melo NC, de Vasconcelos RS. Morphometric and topographic study of coronary ostia. Arq Bras Cardiol 2003;81:359-62, 355-8.
- Waller BF, Orr CM, Slack JD, et al. Anatomy, histology, and pathology of coronary arteries: a review relevant to new interventional and imaging techniques--Part I. Clin Cardiol 1992;15:451-7. [PubMed]
- Ribeiro HB, Sarmento-Leite R, Siqueira DA, et al. Coronary obstruction following transcatheter aortic valve implantation. Arq Bras Cardiol 2014;102:93-6. [PubMed]
- Kapadia SR, Svensson LG, Roselli E, et al. Single center TAVR experience with a focus on the prevention and management of catastrophic complications. Catheter Cardiovasc Interv 2014;84:834-42. [PubMed]
- Amat-Santos IJ, Dahou A, Webb J, et al. Comparison of hemodynamic performance of the balloon-expandable SAPIEN 3 versus SAPIEN XT transcatheter valve. Am J Cardiol 2014;114:1075-82. [PubMed]
- Ribeiro HB, Nombela-Franco L, Urena M, et al. Coronary obstruction following transcatheter aortic valve implantation: a systematic review. JACC Cardiovasc Interv 2013;6:452-61. [PubMed]
- Islam MN, Khan ZI, Khan SR, et al. Morphometry of the intercommissural distances and other structures of the aortic valve of bovine heart. Mymensingh Med J 2006;15:153-8. [PubMed]
- Krishnaswamy A, Parashar A, Agarwal S, et al. Predicting vascular complications during transfemoral transcatheter aortic valve replacement using computed tomography: a novel area-based index. Catheter Cardiovasc Interv 2014;84:844-51. [PubMed]
- Hawkey MC, Lauck SB, Perpetua EM, et al. Transcatheter aortic valve replacement program development: Recommendations for best practice. Catheter Cardiovasc Interv 2014;84:859-67. [PubMed]
- Rivard AL, Bartel T, Bianco RW, et al. Evaluation of aortic root and valve calcifications by multi-detector computed tomography. J Heart Valve Dis 2009;18:662-70. [PubMed]


