Good neurological recovery after rescue thrombolysis of presumed pulmonary embolism despite prior 100 minutes CPR
Introduction
Pulmonary embolism (PE) causes about 5% to 10% of all cardiac arrests (1). Whenever an atraumatic cardiac arrest happened to a previously healthy adult, it is of paramount importance to search for underlying etiology besides the implement of standard cardiopulmonary resuscitation (CPR). Specifically, for cardiac arrest caused by massive PE, an earlier thrombotic therapy based on presumptive diagnosis of PE may significantly improve patients’ outcomes (2). However, it is still a challenge to make the timely diagnosis of PE in clinical practice, especially under the scenario of cardiac arrest. Herein, we reported a 70-year-old man with suddenly cardiac arrest and failed to return of spontaneous circulation (ROSC) after a 100-minute CPR. Then a rescue thrombolytic alteplase saved his life since he was highly suspected to have PE.
Case report
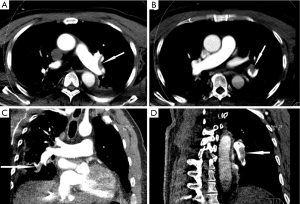
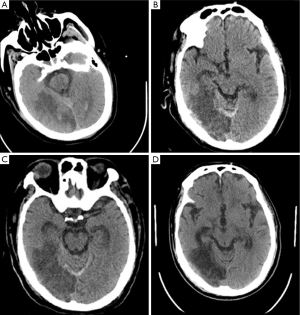
A 70-year-old man was admitted to neurologic department for evaluation of recurrent syncope. He had no associated chest pain, fever, or respiratory symptoms. He was otherwise healthy except a prior MRI demonstrated some minor lacunar infarctions which did not affect his speaking or movement. He was in a sedentary lifestyle after retirement, sitting at least 8 hours every day. The electrocardiogram showed sinus tachycardia with right bundle branch block. On physical examination, he was alert and with mild shortness of breath. Vital signs on presentation included heart rate 105 beats/min, blood pressure 122/78 mmHg, and respiratory rate 16 breaths/min. Oxygen saturation on room air was 95%. Echocardiography demonstrated sizes of four heart chambers were normal and the right ventricular systolic pressure was 63 mmHg. Approximate 2 hours after his arrival in neurologic ward when he was enjoying his dinner, the patient suddenly fell into unconsciousness and the saturation decreased to 90% while the heart rate and respiratory rate increased to 118 beats/min and 48 breaths/min, respectively. Approximate 10 minutes later, he restored his consciousness and complained shortness of breath and the oxygen saturation on room air was 90%. Shortly afterwards, his condition rapidly deteriorated and progressed to cardiopulmonary arrest with pulseless electric activity (PEA). Prompt CPR was initiated and epinephrine 1 mg was administered immediately. Ventricular fibrillation was demonstrated once after approximate 20 minutes CPR, therefore, electrical defibrillations were applied but failed. Thereafter, recurrent narrow QRS complex PEA at a rate around 130 beats per minute dominated the next 60 minutes of CPR, with intermittent ROSC was observed. Given the refractory PEA during the cardiac arrest, potential reversible reasons for cardiac arrest were evaluated as the CPR continued. Another bedside echocardiography demonstrated markedly dilated right ventricle deviating to the left side which significantly depressed the left ventricular systolic function. By incorporating echocardiographic findings, his sedentary lifestyle and D-dimer was 6.03 μg/mL (reference range, 0-0.05 ug/mL), the patient was deemed to have high likelihood of PE. A bolus dose of 5 mg of alteplase was administered and another 45 mg alteplase was given continuously. Approximate 2 minutes after the alteplase infusion, the refractory PEA returned into sinus rhythm and a stable spontaneous circulation was achieved under continuous dopamine support. Overall, the patient underwent approximate 100 minutes of standard CPR. Echocardiography after thrombolysis demonstrated the size of left ventricle was restored although the size of right ventricle was slightly enlarged and the right ventricular systolic pressure was decreased to 45 mmHg. After stabilization and observation for the night, the patient underwent a computed tomography pulmonary angiography which confirmed extensive bilateral pulmonary emboli and no intracranial bleeding (Figures 1 and 2A). The patients had a massive necrosis of intestinal mucosa after thrombolysis and acute kidney injury which necessitated renal replacement treatment for 3 months. During the following week, constant heparin infusion was administered and later was shifted to warfarin for anticoagulation. One week later, after withdrawn of sedation, the patient became awake and alert. Another head CT scan performed 6 days after thrombolysis demonstrated ischemic infarction and mild intracranial bleeding in the right occipital lobe (Figure 2B). However, the heparin was continued and a third head CT at 10 days post thrombolysis did not show enlarging intracranial hemorrhage (Figure 2C). Currently, the patient is alert and is on a physical rehabilitation program but no major neurological sequelae related to his cardiac arrest (Figure 2D).
Discussion
Mortality due to cardiac arrest following massive PE ranges from 65% to 95% (3). As a result of persisting mechanical obstruction by PE, routine CPR is often ineffective. Given the poor outcome of prolonged unresponsive cardiac arrest and the fact that vast majority of cardiac arrest were caused by acute myocardial infarction or PE, empirical thrombolytic therapy seems to be intuitive appeal for cardiac arrests. However, a recent study from New England Journal of Medicine demonstrated that unselected thrombolysis for cardiac arrest patients was not all beneficial (4). Nevertheless, both the American Heart Association and European Resuscitation Council recommend “considering” use of fibrinolytic therapy when cardiac arrest is caused by proven or suspected acute PE (5,6). In patients presenting with cardiac arrest as a consequence of acute PE, thrombolytic therapy was associated with significantly higher ROSC (81% vs. 43%, P=0.03) (7). Overall, fibrinolysis reduced the risk of death by around 55% (8). In a recent study by Er et al. (9) shown that for cardiac arrests caused by PE, earlier initiated thrombosis after cardiac arrest onset (13.6 vs. 24.6 min) was associated with higher rate of ROSC eventually. Moreover, for those who were eventually discharged, thrombolysis was given with less delay (11.0 vs. 22.5 min). Therefore, the earlier rescue thrombolysis was administrated for patients with cardiac arrest and presumptive diagnosis of PE, the better outcome it would be. In our case, however, the thrombolysis was somewhat delayed because the relative inexperience of neurologic physicians who were involved in the primary resuscitation and the CPR continued for nearly 100 minutes overall until the presumptive diagnosis was established by the consulted intensive care physician. Given an approximate 30 minutes of CPR is adopted by many physicians for cardiac arrests currently, a longer time of CPR may be associated with higher rate of ROSC, especially for patients caused by massive PE and received a timely thrombolytic therapy.
In fact, it could be extremely difficult to explore the etiology of cardiac arrest while a patient is being resuscitated, specifically for physicians outside ICU. However, certain physiologic precepts have a stronger association with PE, lending confidence to presumptive diagnosis in the absence of evident alternative etiologies (2). For example, a rapid, narrow QRS PEA rhythm with observed myocardial contraction on echocardiography may have a higher association with PE (10). A triad of witnessed cardiac arrest, age less than 65 to 70 years, and PEA as the initial rhythm was seemed to be more likely associated with massive PE (11,12). The following factors were proved to have the highest association with the diagnosis of PE: unilateral leg swelling, oxygen saturation less than 95%, active cancer, recent prolonged immobilization or orthopedic surgery (2). Importantly, a high index of suspicion for PE should be maintained especially in cardiac arrest patient who had a history or symptom profile associated with this diagnosis. In clinical practice, a rapid integration of patients’ information, bedside diagnostic tools and available laboratory findings could be relied on to determine the level of suspicion of PE. With respect to the diagnostic tool, a portable echocardiography is ideal because it can differentiate shock categories and recognize the characteristics of PE even during CPR. Moreover, transesophageal echocardiography can reliably establish the cause of a circulatory arrest during CPR (13,14). However, for those who can be stabilized, a computed tomography pulmonary angiography will demonstrate filling defects in the main or lobar pulmonary arteries. As mentioned, we made the presumptive diagnosis of PE by integrating the patient’s lifestyle, elevated D-dimer, and findings of bedside echocardiography.
Given the bleeding risk associated with thrombolytic therapy, this intervention should only be administrated after balancing the potential benefits of improved outcomes and bleeding risk. Of the potential bleeding risk, the intracranial hemorrhage is the most devastating complication (2). Major bleeding was reported to occur in 9.6% of patients receiving thrombolytic therapy for acute PE while intracranial hemorrhage reported to be approximate 3% (15). There was a mild bleeding after thrombolysis in our case, however, no enlargement was noticed even under constant infusion of heparin for anticoagulation for the PE. Nevertheless, risk stratification models for bleeding are warranted to identify the individuals at the highest risk of hemorrhagic complications. In the recent large Pulmonary Embolism Thrombolysis (PEITHO) trial, incidence of major bleeding and intracranial hemorrhage was 6.3% and 2% respectively (16). Interestingly, bleeding risk was lower and mortality benefit was higher in patients <75 years. This is consistent with a recent meta-analysis that although use of thrombolytics was associated with increased risks of major bleeding and intracranial hemorrhage, major bleeding was not significantly increased in patients 65 years and younger (17). Hence, attenuation of the bleeding risk in individuals 75 years and younger may suggest a stronger indication for thrombolysis in those patients. Moreover, a risk score based on six variables (age >75 years, recent bleeding, cancer, creatinine levels >1.2 mg/dL, anemia, or PE at baseline) documented at entry can identify patients with venous thromboembolism at low, intermediate, or high risk for major bleeding during the first three months of therapy (18). However, given the outcomes of cardiac arrest due to PE are uniformly poor and thrombolysis therapy is generally deemed as ‘‘last chance’’ under many circumstances, even for patient with contraindication such as recent intracranial hemorrhage, a good outcome could be achieved (8). Under this circumstance, contraindications of thrombolytic therapy are frequently disregarded because the potential benefit outweighs the bleeding risk.
In summary, early recognition of etiology is of paramount importance for successful resuscitation of patients with atraumatic cardiac arrest. For cardiac arrest caused by massive PE, a good neurological recovery could be achieved by rescue thrombolysis despite a prolonged 100 minutes CPR.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Adams BD, Kim JY, Jackson WO. Tenecteplase and return of spontaneous circulation after refractory cardiopulmonary arrest. South Med J 2004;97:1015-7. [PubMed]
- Logan JK, Pantle H, Huiras P, et al. Evidence-based diagnosis and thrombolytic treatment of cardiac arrest or periarrest due to suspected pulmonary embolism. Am J Emerg Med 2014;32:789-96. [PubMed]
- Bailén MR, Cuadra JA, Aguayo De Hoyos E. Thrombolysis during cardiopulmonary resuscitation in fulminant pulmonary embolism: a review. Crit Care Med 2001;29:2211-9. [PubMed]
- Böttiger BW, Arntz HR, Chamberlain DA, et al. Thrombolysis during resuscitation for out-of-hospital cardiac arrest. N Engl J Med 2008;359:2651-62. [PubMed]
- Morrison LJ, Deakin CD, Morley PT, et al. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2010;122:S345-421. [PubMed]
- Deakin CD, Nolan JP, Soar J, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation 2010;81:1305-52. [PubMed]
- Kürkciyan I, Meron G, Sterz F, et al. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med 2000;160:1529-35. [PubMed]
- Bottinor W, Turlington J, Raza S, et al. Life-saving systemic thrombolysis in a patient with massive pulmonary embolism and a recent hemorrhagic cerebrovascular accident. Tex Heart Inst J 2014;41:174-6. [PubMed]
- Er F, Nia AM, Gassanov N, et al. Impact of rescue-thrombolysis during cardiopulmonary resuscitation in patients with pulmonary embolism. PLoS One 2009;4:e8323. [PubMed]
- Zhu T, Pan K, Shu Q. Successful resuscitation with thrombolysis of a presumed fulminant pulmonary embolism during cardiac arrest. Am J Emerg Med 2013;31:453.e1-3.
- Courtney DM, Sasser HC, Pincus CL, et al. Pulseless electrical activity with witnessed arrest as a predictor of sudden death from massive pulmonary embolism in outpatients. Resuscitation 2001;49:265-72. [PubMed]
- Courtney DM, Kline JA. Prospective use of a clinical decision rule to identify pulmonary embolism as likely cause of outpatient cardiac arrest. Resuscitation 2005;65:57-64. [PubMed]
- Dirican A, Ozkaya S, Atas AE, et al. Thrombolytic treatment (alteplase; rt-Pa) in acute massive pulmonary embolism and cardiopulmonary arrest. Drug Des Devel Ther 2014;8:759-63. [PubMed]
- van der Wouw PA, Koster RW, Delemarre BJ, et al. Diagnostic accuracy of transesophageal echocardiography during cardiopulmonary resuscitation. J Am Coll Cardiol 1997;30:780-3. [PubMed]
- Hefer DV, Munir A, Khouli H. Low-dose tenecteplase during cardiopulmonary resuscitation due to massive pulmonary embolism: a case report and review of previously reported cases. Blood Coagul Fibrinolysis 2007;18:691-4. [PubMed]
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402-11. [PubMed]
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014;311:2414-21. [PubMed]
- Ruíz-Giménez N, Suárez C, González R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost 2008;100:26-31. [PubMed]


