
Oral antiplatelet therapy in the elderly undergoing percutaneous coronary intervention: an umbrella review
It’s not how old you are, it’s how you are old
——Jules Renard
Introduction
The management of ischemic heart disease in general and coronary artery disease in particular has been recently revolutionized by concomitant breakthroughs in pathophysiologic insight, prevention, diagnosis, risk stratification, treatment and rehabilitation (1,2). Focusing specifically on management, the advent and refinement of percutaneous coronary intervention has proved hugely beneficial to patients thanks to the mirroring refinements in antithrombotic therapy (2,3). At the beginning of the percutaneous coronary intervention era potent anticoagulants were the rule before, during as well as after percutaneous coronary intervention, with ensuing high bleeding risk despite the paradox of a suboptimal prevention of acute and subacute thrombosis (4,5). Luckily enough, the central role of platelets in causing stent thrombosis was recognized eventually, and thus dual antiplatelet regimens became the key pharmacologic underpinning of percutaneous coronary intervention (6). Nowadays, percutaneous coronary intervention rests solidly on minimally invasive access (i.e., radial), simple and straightforward techniques for lesion preparation and stent implantation, followed by careful optimization of acute results (7). Devices for percutaneous coronary intervention are quite refined, with new-generation drug-eluting stents yielding very low risks of restenosis and thrombosis (8-10). Accordingly, practitioners have witnessed major developments in oral antiplatelet therapy for patients undergoing percutaneous coronary intervention, including molecule type (ranging from aspirin to cilostazol, clopidogrel, dipyridamole, prasugrel, ticagrelor and ticlopidine), front-loading strategy, maintenance dose, regimen duration, escalation, de-escalation, and combination (11). On top of this, oral anticoagulants (ranging from warfarin to apixaban, dabigatran, edoxaban and rivaroxaban) can be added to a single oral antiplatelet regimen or even to a dual antiplatelet regimen, in a framework of triple antithrombotic therapy (12). While this scenario might seem complicated at first glance, we should be reminded by ornithological principles that birds do not fly simply because they have wings, but because they go around flapping them. Accordingly, percutaneous coronary intervention successes in the individual patient strongly rest on picking for each one the most appropriate antiplatelet management strategy.
This approach indeed applies to all patients, irrespective of their age, but it is clear that elderly subjects represent a unique group, given their increased risk of adverse events as well as complications, their frequent comorbidities, and their suboptimal compliance and adherence to prescribed regimens (13,14). Most importantly, elderly patients (especially when focusing on those older than 75 or more) are typically underrepresented in clinical trials, thus requiring complex decision making based on extrapolation when planning the best antiplatelet therapy regimen before, during and after percutaneous coronary intervention (15-19).
We hereby provide a comprehensive overview of oral antiplatelet management strategy for patients undergoing percutaneous coronary intervention, with a specific focus on subjects with advanced age.
Reviewing methods
For the purpose of this review, we opted for an umbrella review design with scoping purposes (20-22). Briefly, PubMed was searched for suitable systematic reviews using the following string: (elderly OR octogenarian* OR octagenarian* OR nonagenarian OR aged OR old* OR (age AND (advanced OR higher OR older))) AND (antiplatelet* OR aspirin OR dipyridamole OR ticagrelor OR clopidogrel OR prasugrel OR ticlopidine OR cilostazol) AND ((percutaneous AND coronary AND intervention) OR ptca) AND systematic[sb]. Reviews were selected if reporting an original systematic review of clinical trials or observational studies on antiplatelet therapy for patients undergoing percutaneous coronary intervention and focusing, at least in part, on elderly subjects (i.e., those aged 65 years or more). No language restriction was enforced. Salient details on reviews, included studies and patients, interventions, and outcomes were collected. Finally, review quality was appraised using the Oxman and Guyatt Overview Quality Assessment Questionnaire (OQAQ) (21).
Main findings
Our dedicated systematic review initially retrieved 25 citations, and eventually, after excluding all non-pertinent or evidently duplicate ones at the title or abstract level, we collected 8 systematic reviews (Table 1) (23-30). They ranged from systematic reviews without statistical synthesis to meta-analyses and umbrella reviews, including in some instances only non-randomized controlled trials, in others only randomized trials, or both. The total number of included studies was high as 137 in the most comprehensive review, yielding as many as 313,460 patients being overviewed. In terms of validity, most reviews were of high validity, despite some noticeable drawbacks, especially in terms of search or pooling strategy (Table 2).
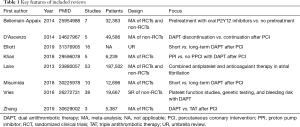
Full table
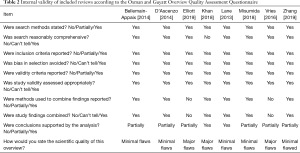
Full table
In particular, Vries et al. conducted a thorough systematic review on 38 observational studies including 19,667 patients receiving dual antiplatelet therapy who had undergone platelet function studies, genetic testing, or appraisal of bleeding risk (29). They found that the risk of bleeding could be predicted by identifying low on-treatment platelet reactivity by means of several different platelet function tests, by recognizing carriage of the CYP2C19*17 allele, and by using a bleeding score such as the RISK-PCI or ISTH/SSC ones. Notably, in most scores age (either appraised as a continuous variable or as a discrete one when >70–75 years) proved a key component of bleeding scores. Bellemain-Appaix and colleagues pooled 7 randomized and non-randomized clinical trials including as many as 32,383 patients with non-ST-elevation acute coronary syndromes assigned to pretreatment with oral P2Y12 inhibitors vs. no pretreatment (23). They found that pretreatment with potent oral antiplatelet agents was not associated with significant changes in mortality, but caused a significant increase in the risk of bleeding.
Our group, under the leadership of Fabrizio D’Ascenzo, pooled data from 5 observational studies, including 49,586 patients with acute coronary syndromes (24). We focused on the impact of discontinuation of dual antiplatelet therapy after 12 months of uninterrupted assumption. Notably, discontinuation after 12 months appeared safe in subjects managed medically, whereas it was associated with more thrombotic events in those undergoing percutaneous coronary intervention with stent implantation. On the contrary, Misumida et al. pooled 10 randomized clinical trials comparing short-term (3–6 months) dual antiplatelet therapy vs. long-term dual antiplatelet therapy (12–24 months) in 12,696 patients with acute coronary syndromes undergoing percutaneous coronary intervention, most receiving clopidogrel and second-generation drug-eluting stents (28). They found actually that both thrombotic and bleeding events occurred with similar rates irrespective of dual antiplatelet therapy duration, despite trends in favor of long-term regimens to prevent stent thrombosis, and in favor of short-term regimens to reduce the risk of bleeding. Elliott et al. conducted an umbrella review quite similar in design to our present work, but different in terms of scope and focus (25). Specifically, they selected 16 systematic reviews appraising the risk-benefit balance of long-term dual antiplatelet therapy after percutaneous coronary intervention, albeit including only 8 randomized trials. They concluded that prolonging dual antiplatelet therapy beyond 1 year may reduce the risk of myocardial infarction and stent thrombosis, but may increase the risk of death and major bleeding, especially in subjects at higher risk of bleeding. Lane and colleagues published in 2013 a detailed systematic review on the combination of oral anticoagulants and oral antiplatelet agents in patients with atrial fibrillation and high-risk features (including thus coronary artery disease), totaling 53 randomized and non-randomized studies and 187,502 patients (27). They concluded that at that time there was no evidence in favor of combination therapy in such condition. However, subsequently dedicated trials have been published and should be taken into account (Table 3). A similar focus was found in the work by Zhang and colleagues, but their restrictive selection criteria led to the inclusion of only 3 randomized trials and 5,387 patients receiving either dual antithrombotic therapy (i.e., a regimen including an oral anticoagulant and an antiplatelet agent, typically omitting aspirin) or triple antithrombotic therapy (i.e., a regimen including an oral anticoagulant and two antiplatelet agents) (30). They found that overall dual antithrombotic therapy was associated with similar rates of thrombotic events but lower rates of bleedings in comparison to triple therapy. However, when analyzing in details elderly patients, a potential increase in atherothrombotic events partially offset the reduction in bleedings associated with dual antithrombotic therapy. Finally, Khan et al. conducted a meta-analysis on 5 randomized clinical trials including 6,239 patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction, who had been randomized to dual antiplatelet therapy plus proton pump inhibitors vs. dual antiplatelet therapy alone (26). While proton pump inhibitors did not impact adversely on thrombotic events, their use was associated with significant reductions in the risk of gastrointestinal bleeding, gastrointestinal ulcers, and gastrointestinal erosions. In addition, an intriguing trend toward fewer episodes of post-revascularization unstable angina was found favoring the gastroprotection group.
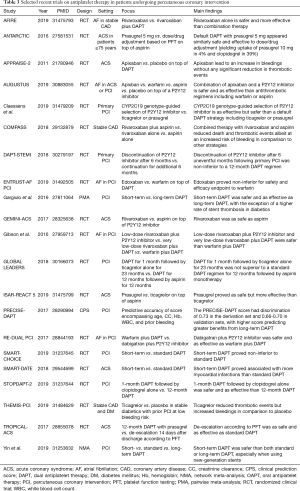
Full table
Implications for patient care
The evidence so far accrued, ranging from the systematic reviews described above to the many recent trials completed on antithrombotic therapy in patients undergoing percutaneous coronary intervention, poses several major challenges to practitioners taking care of elderly subjects in whom this procedure is envisioned, as well as in those who have completed it successfully, more or less recently (13,31). Indeed, uncertainties on prediction, pretreatment, loading, genetic testing, individual agent, combination, functional testing, duration, escalation/de-escalation, and adherence persist (32-35). These challenges skyrocket when we envision the concomitant presence of comorbidities or other conditions, such as atrial fibrillation, and the unique features of the available antiplatelet agents (Table 4).
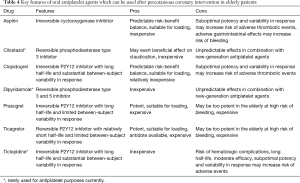
Full table
A pragmatic approach rests in our opinion, and according to several experts, on refined framing of patient features, and comprehensive decision making encompassing diagnosis, risk-stratification, prognostication, warranty period definition, and invasive assessment (36-38). Once the decision to proceed with percutaneous coronary intervention is made, the choice of antiplatelet therapy should occur together with the technical planning of the revascularization procedure. Furthermore, the initial treatment plan should be periodically reviewed, allowing for escalation, de-escalation, interruption, or prolongation of the chosen antiplatelet regimen (39).
Briefly, subjects at low risk of bleeding and low risk of thrombotic events could be managed with a relatively short (e.g., 6–12 months) dual antiplatelet regimen, whereas subjects at high bleeding risk (e.g., those receiving also anticoagulant therapy) probably need even shorter regimens (e.g., 1–3 months) (40,41). Patients at high thrombotic risk and low bleeding risk can be considered eligible for refined escalation/de-escalation regimens (e.g., substituting dual antiplatelet therapy with monotherapy based on P2Y12 inhibitor), or default long-term dual antiplatelet therapy (e.g., 12 months or more), with the explicit provision that such indication can be revised if necessary or indicated by functional or genetic testing (42). Finally, dual antithrombotic therapy with a novel oral anticoagulant and a P2Y12 inhibitor is clearly the most appealing strategy in terms of risk-benefit when atrial fibrillation coexists.
Conclusions
When considering oral antiplatelet therapy for elderly patients undergoing percutaneous coronary intervention, several drugs are available, ranging from aspirin to cilostazol, clopidogrel, dipyridamole, prasugrel, ticagrelor, and ticlopidine. Yet, most commonly a dual antiplatelet therapy comprised of aspirin and a P2Y12 inhibitor is recommended, with subtle adjustments for pretreatment, loading, dose, duration, escalation or de-escalation, with the potential adjunct in selected patients of novel oral anticoagulants. Indeed, a flexible and individualized approach to oral antiplatelet therapy in elderly patients undergoing percutaneous coronary intervention is paramount, factoring in patient features (exploiting thrombotic, bleeding and frailty scores), triage, angiographic and other imaging features, interventional technique, stent choice, rehabilitation, and secondary prevention.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ion S. Jovin) for the series “Interventional Cardiology” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2019.12.87). The series “Interventional Cardiology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Biondi-Zoccai GG, Abbate A, Liuzzo G, et al. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003;41:1071-7. [Crossref] [PubMed]
- Piccolo R, Giustino G, Mehran R, et al. Stable coronary artery disease: revascularisation and invasive strategies. Lancet 2015;386:702-13. [Crossref] [PubMed]
- Biondi-Zoccai GG, Abbate A, Agostoni P, et al. Long-term benefits of an early invasive management in acute coronary syndromes depend on intracoronary stenting and aggressive antiplatelet treatment: a metaregression. Am Heart J 2005;149:504-11. [Crossref] [PubMed]
- Giordano A, Musumeci G, D’Angelillo A, et al. Effects of Glycoprotein IIb/IIIa Antagonists: Anti Platelet Aggregation and Beyond. Curr Drug Metab 2016;17:194-203. [Crossref] [PubMed]
- D'Ascenzo F, Taha S, Moretti C, et al. Meta-analysis of randomized controlled trials and adjusted observational results of use of clopidogrel, aspirin, and oral anticoagulants in patients undergoing percutaneous coronary intervention. Am J Cardiol 2015;115:1185-93. [Crossref] [PubMed]
- Capranzano P, Angiolillo DJ. Tailoring P2Y12 inhibiting therapy in elderly patients with myocardial infarction undergoing primary percutaneous coronary intervention. J Am Heart Assoc 2019;8:e014000. [Crossref] [PubMed]
- Agostoni P, Zuffi A, Faurie B, et al. Same wrist intervention via the cubital (ulnar) artery in case of radial puncture failure for percutaneous cardiac catheterization or intervention: the multicenter SWITCH registry. Int J Cardiol 2013;169:52-6. [Crossref] [PubMed]
- Biondi-Zoccai GG, Agostoni P, Abbate A, et al. Adjusted indirect comparison of intracoronary drug-eluting stents: evidence from a metaanalysis of randomized bare-metal-stent-controlled trials. Int J Cardiol 2005;100:119-23. [Crossref] [PubMed]
- Capranzano P, Sanfilippo A, Tagliareni F, et al. Long-term outcomes after drug-eluting stent for the treatment of ostial left anterior descending coronary artery lesions. Am Heart J 2010;160:973-8. [Crossref] [PubMed]
- Giordano A, Polimeno M, Corcione N, et al. Synergy between direct coronary stenting technique and use of the novel thin strut cobalt chromium SkylorTM stent: the MACE in follow up patients treated with Skylortm stent (MILES STUDY). Curr Cardiol Rev 2012;8:6-13. [Crossref] [PubMed]
- Palmerini T, Della Riva D, Benedetto U, et al. Three, six, or twelve months of dual antiplatelet therapy after DES implantation in patients with or without acute coronary syndromes: an individual patient data pairwise and network meta-analysis of six randomized trials and 11 473 patients. Eur Heart J 2017;38:1034-43. [PubMed]
- Shlofmitz E, Shlofmitz R, Lee MS. The Role of Novel Oral Anticoagulants and Antiplatelet Therapy after Percutaneous Coronary Intervention: Individualizing Therapy to Optimize Outcomes. Korean Circ J 2019;49:645-56. [Crossref] [PubMed]
- Andreotti F, Rocca B, Husted S, et al. ESC Thrombosis Working Group. Antithrombotic therapy in the elderly: expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J 2015;36:3238-49. [PubMed]
- Nudi F, Biondi-Zoccai G, Schillaci O, et al. Prognostic accuracy of myocardial perfusion imaging in octogenarians. J Nucl Cardiol 2018;25:1342-9. [Crossref] [PubMed]
- Savonitto S, Morici N, De Servi S. Antiplatelet therapy for elderly patients with acute coronary syndromes. Aging (Albany NY) 2018;10:2220-1. [Crossref] [PubMed]
- Schoenenberger AW, Radovanovic D, Windecker S, et al. AMIS Plus Investigators. Temporal trends in the treatment and outcomes of elderly patients with acute coronary syndrome. Eur Heart J 2016;37:1304-11. [Crossref] [PubMed]
- Presutti DG, D'Ascenzo F, Omedè P, et al. Percutaneous coronary intervention in nonagenarian: a meta-analysis of observational studies. J Cardiovasc Med (Hagerstown) 2013;14:773-9. [Crossref] [PubMed]
- Biondi Zoccai G, Abbate A, D'Ascenzo F, et al. Percutaneous coronary intervention in nonagenarians: pros and cons. J Geriatr Cardiol 2013;10:82-90. [PubMed]
- Sillano D, Moretti C, Biondi-Zoccai G, et al. Percutaneous unprotected left main angioplasty with drug-eluting stents in a nonagenarian: feasible and safe despite recurrent restenosis. Minerva Cardioangiol 2008;56:167-70. [PubMed]
- Biondi-Zoccai G. editor. Network Meta-Analysis: Evidence Synthesis with Mixed Treatment Comparison. Hauppauge, NY: Nova Science Publishers, 2014.
- Biondi-Zoccai G. editor. Umbrella Reviews: Evidence Synthesis with Overviews of Reviews and Meta-Epidemiologic Studies. Cham: Springer International Publishing, 2016.
- Biondi-Zoccai G. editor. Diagnostic Meta-Analysis: A Useful Tool for Clinical Decision-Making. Cham: Springer International Publishing, 2018.
- Bellemain-Appaix A, Kerneis M, O'Connor SA, et al. Reappraisal of thienopyridine pretreatment in patients with non-ST elevation acute coronary syndrome: a systematic review and meta-analysis. BMJ 2014;349:g6269. [Crossref] [PubMed]
- D'Ascenzo F, Colombo F, Barbero U, et al. Discontinuation of dual antiplatelet therapy over 12 months after acute coronary syndromes increases risk for adverse events in patients treated with percutaneous coronary intervention: systematic review and meta-analysis. J Interv Cardiol 2014;27:233-41. [Crossref] [PubMed]
- Elliott J, Kelly SE, Bai Z, et al. Optimal Duration of Dual Antiplatelet Therapy Following Percutaneous Coronary Intervention: An Umbrella Review. Can J Cardiol 2019;35:1039-46. [Crossref] [PubMed]
- Khan MY, Siddiqui WJ, Alvarez C, et al. Reduction in postpercutaneous coronary intervention angina in addition to gastrointestinal events in patients on combined proton pump inhibitors and dual antiplatelet therapy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2018;30:847-53. [Crossref] [PubMed]
- Lane DA, Raichand S, Moore D, et al. Combined anticoagulation and antiplatelet therapy for high-risk patients with atrial fibrillation: a systematic review. Health Technol Assess 2013;17:1-188. [Crossref] [PubMed]
- Misumida N, Abo-Aly M, Kim SM, et al. Efficacy and safety of short-term dual antiplatelet therapy (≤6 months) after percutaneous coronary intervention for acute coronary syndrome: A systematic review and meta-analysis of randomized controlled trials. Clin Cardiol 2018;41:1455-62. [Crossref] [PubMed]
- Vries MJ, van der Meijden PE, Henskens YM, et al. Assessment of bleeding risk in patients with coronary artery disease on dual antiplatelet therapy. A systematic review. Thromb Haemost 2016;115:7-24. [Crossref] [PubMed]
- Zhang J, Wang Z, Sang W, et al. Omission of aspirin in patients taking oral anticoagulation after percutaneous coronary intervention: a systematic review and meta-analysis. Coron Artery Dis 2019;30:109-15. [Crossref] [PubMed]
- Leonardi S, Bueno H, Ahrens I, et al. Optimised care of elderly patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2018;7:287-95. [Crossref] [PubMed]
- Biondi-Zoccai G, Lotrionte M, Agostoni P, et al. Adjusted indirect comparison meta-analysis of prasugrel versus ticagrelor for patients with acute coronary syndromes. Int J Cardiol 2011;150:325-31. [Crossref] [PubMed]
- Costa F, van Klaveren D, James S, et al. PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025-34. [Crossref] [PubMed]
- Schüpke S, Neumann FJ, Menichelli M, et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Cayla G, Cuisset T, Silvain J, et al. ANTARCTIC investigators. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016;388:2015-22. [Crossref] [PubMed]
- Nudi F, Lotrionte M, Biasucci LM, et al. Comparative safety and effectiveness of coronary computed tomography: Systematic review and meta-analysis including 11 randomized controlled trials and 19,957 patients. Int J Cardiol 2016;222:352-8. [Crossref] [PubMed]
- Tegn N, Abdelnoor M, Aaberge L, et al. After Eighty study investigators. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomised controlled trial. Lancet 2016;387:1057-65. [Crossref] [PubMed]
- Riobóo-Lestón L, Raposeiras-Roubin S, Abu-Assi E, et al. Bleeding risk assessment in elderly patients with acute coronary syndrome. J Geriatr Cardiol 2019;16:145-50. [PubMed]
- Zhao G, Zhou M, Ma C, et al. In-Hospital Outcomes of Dual Loading Antiplatelet Therapy in Patients 75 Years and Older With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention: Findings From the CCC-ACS (Improving Care for Cardiovascular Disease in China-Acute Coronary Syndrome) Project. J Am Heart Assoc 2018;7:e008100. [Crossref] [PubMed]
- De Rosa R, Piscione F, Galasso G, et al. Antiplatelet therapy in very elderly and comorbid patients with acute coronary syndromes. J Geriatr Cardiol 2019;16:103-13. [PubMed]
- Benedetti G, Neccia M, Agati L. Direct oral anticoagulants use in elderly patients with non valvular atrial fibrillation: state of evidence. Minerva Cardioangiol 2018;66:301-13. [PubMed]
- Erathi HV, Durgaprasad R, Velam V, et al. Evaluation of On-Clopidogrel platelet reactivity overtime, SYNTAX SCORE, genetic polymorphisms and their relationship to one year clinical outcomes in STEMI patients undergoing PCI. Minerva Cardioangiol 2018;66:16-25. [PubMed]


