Rapid pleurodesis is an outpatient alternative in patients with malignant pleural effusions: a prospective randomized controlled trial
Introduction
The management of patients with malignant and/or recurrent pleural effusions is cumbersome and can present important diagnostic and therapeutic challenges (1,2).
Pleural effusion causing symptoms such as chest pain and dyspnea is a common problem that causes significant morbidity and can negatively affect quality of life of patients for their remaining months. Despite management of underlying malignancy with chemo/radiotherapy, malignant pleural effusions (MPE) may persist or recur and necessitate palliative interventions in order to control or alleviate the symptoms. Several palliative treatment options are available including therapeutic thoracentesis, tube thoracostomy, chemical pleurodesis, video thoracoscopic pleurodesis and pleuroperitoneal shunt (2,3).
Pleurodesis is performed to inflame the visceral and parietal pleura to fuse the pleura together obliterating the potential pleural space. Pleurodesis with sclerosing agents has a high success rate but requires hospitalization for up to 6 days until chest tubes can be removed (4). It is generally considered standard treatment for recurrent MPE. Asbestos-free talc has been established as the most effective agent for pleurodesis (4). However, various factors have impact on the success of pleurodesis include initial drainage time, chest drain diameter, management of the chest drain (suction, no suction), etc. (5). Success and length of stay of the patients (5-7 days) are of utmost importance since patients with MPE are usually critically ill and/or moribund. In these patients with an expected survival of only 8 months, the aim of a palliative intervention should be reliable alleviation of dyspnea, improving quality of life, shortening hospital stay as much as possible and keeping the duration of chest tube drainage as short as possible. Traditional catheter drainage of MPE includes tube insertion, daily observation and pleurodesis if daily drainage is <200-400 mL and re-expansion of the lung is provided. However, this approach may lead to very long hospitalization time with limited success of the procedure. We investigated the value and effectiveness of a rapid and pleurodesis method using talc in patients with potentially recurrent pleural effusion. Our secondary hypothesis was whether rapid pleurodesis would decrease the length of hospitalization or not.
Materials and methods
A prospective, randomised method was utilized to compare standard method of pleurodesis with the proposed new rapid pleurodesis process in patients with symptomatic MPE. This is the ‘non-inferiority’ trial of ‘rapid-pleurodesis’ that is to prove that ‘rapid-pleurodesis’ methodology is equally effective to standard procedures with a number of advantages. The institutional review board for human studies has approved the treatment protocols. Type-I (α) error and type-II (β) error were set to 5%. Our non-inferiority margin was 40% difference with a δ0 value of 10%. With a one-sided alternative, we needed to include 40 patients in each arm.
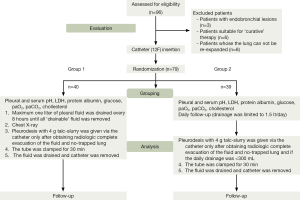
From April 2011 to December 2012, 96 symptomatic patients who had ‘potentially’ recurrent histologically and/or cytologically proven MPE were evaluated. The patients whose lungs did not expand (n=8), patients with endobronchial lesions (n=3), and ones who are suitable for curative therapy were excluded from the randomization. Of those, 79 patients were amenable to be analyzed (36 patients were allocated in each group). There were 37 men, 42 women. The mean age was 58.8 years (range, 25 to 92 years; standard deviation, 16.32 years). Randomization was performed according to an internet based-random number generator. We also aimed to analyze the effect of rapid pleurodesis on hospital stay as secondary goal of the study. A total of 40 patients were randomized into the rapid-pleurodesis arm whereas 39 patients had standard drainage and pleurodesis (control group).
Thoracic drainage and pleurodesis: using local anesthesia (bupivacaine) and intramuscular preemptive analgesia (ketorolac), a small-bore (12F) thoracic catheter was inserted into the pleural space in the posterior axillary line. Study group (group 1): pleural space was drained using valve system connected to the thoracic catheter. One liter of pleural fluid was drained every 8 h until all ‘drainable’ fluid was removed. Pleural space drainage was subsequently evaluated using chest radiographs obtained 2 h after the first drainage and the one taken after the last drainage. Pleurodesis with 4 gr. talc (Steritalc; Novatech; La Ciotat; France) slurry was initiated only after obtaining radiographic evidence of the complete evacuation of the fluid and no-trapped lung. In control group (group 2), controlled drainage was done limited to 1 lt at once and maximum 1.5 lt of fluid was drained per day. Chest radiograph was taken in 2 h. Daily follow-up was performed. Pleurodesis with 4 gr talc slurry was initiated only after obtaining radiographic evidence of the complete evacuation of the fluid, if the daily drainage <300 mL. The tube was clamped for 30 min in all patients (group 1 and group 2). Catheter was withdrawn 1 h after the talc-slurry drainage. Pleural fluid LDH, protein, pH, glucose, pleural fluid amylase, cholesterol, arterial blood partial gas pressure, cytopathological, bacteriological analyses were performed in almost all patients. Informed consent was obtained from all patients before their participation in the study. The Institutional Review Board approved the study before its commencement. The randomized trial was in accordance with the 2001 checklist of the Consolidated Standards of Reporting Trials (CONSORT) Statement. The CONSORT recommended flow diagram of patients is shown in Figure 1. Patients were followed up with plain chest radiographs at 1, 2, 3 and 6 months (if the patient was alive) after pleurodesis. Responses were classified as: (I) complete (no clinical or radiological recurrence of pleural effusion); (II) partial (small amount of fluid re-accumulation in the chest radiograph, but no symptoms); (III) failure (reaccumulation of fluid causing symptoms or needing thoracentesis) (6). The assessment of success was performed by an investigator blinded to allocation.
The mean follow-up of patients was 5.3 months (range, 1 to 18 months). SPSS 15.0 statistical software program was used in data analysis. Student t-test, paired t-test, covariance analysis, Fisher exact test were carried out. The difference was deemed significant when P<0.05.
Results
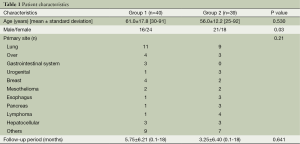
The causes of pleural effusions are shown in Table 1. Demographic and primary disease characteristics are summarized in Table 1. The majority of patients had breast cancer, lung cancer or mesothelioma.
Full table
No-complication developed due to talc-slurry in two groups. Four patients (4.8%) developed sub-febrile fever with an elevated white-blood-cell count requiring antibiotics within 48 h of the pleurodesis. Complete or partial response was achieved in 35 (87.5%) and 33 (84.6%) patients in group 1 and group 2 respectively (P=0.670). The mean total drainage was 2,703 mL in group 1, whereas it was 4,329 mL in group 2 (P=0.016). The mean drainage time was 40.7 and 165.2 h in group 1 and group 2 respectively (P<0.001).
The mean length of stay was 2.2 in group 1, whereas it was 9.0 days in group 2 (P<0.001). In rapid-pleurodesis group, the procedure was completed in less than 24 h in 32 out of 40 patients (80.0%). We found no predictive factor indicating success in all patients (Table 2).

Full table
Discussion
Malignant and/or recurrent pleural effusions are associated with significant morbidity.
The key point to achieve successful pleurodesis is the ability to fully drain the pleural space and to re-expand the lung. Prompt clinical evaluation followed by aggressive treatment often results in successful palliation (1). Treatment response for MPE is highly variable (2). We have developed a new method of rapid pleurodesis, which worked effectively in patients with short survival expectations. The selection of pleurodesis agents stays debatable. Talc is more effective, but is allied with more unfavorable effects. Talc pleurodesis is followed by systemic and pulmonary inflammation (5,7). Spiegler published a rapid pleurodesis series with considerable success, shorter hospital stay and possibly lower cost (8). They found that, chemical pleurodesis can be accomplished with good results in the majority of patients (complete obliteration: 48%, partial response: 31%) with bleomycin in less 1 day.
Yildirim et al. preferred to use oxytetracycline for rapid pleurodesis (9). They utilized a very similar rapid pleurodesis methodology. Differently, we drained all pleural fluid before talc-slurry implementation. They advocated oxytetracycline since it was well tolerated by the patients without major side effects (9). In our series, we did not observe any serious side effect possibly attributable to talc. Janssen et al. reported that, large-particle size (22.5 µm) talc for pleurodesis is safer and not associated with the development of acute respiratory distress syndrome (10). We used the same particle-size and brand talc. We found that, rapid pleurodesis protocol with talc is safe and effective. It also led to shorter hospital stay and possibly low-hospital cost. It is reasonable to suggest that, fast and effective pleurodesis may have caused less hospital-related morbidity. In the study, the pleural fluid was aspirated manually at 8 h intervals to keep both pleura in close apposition before instillation of talc. The main purpose was to increase the contact duration and surface area of both pleura for increasing the chemical pleuritis in the shortest possible interval.
Reddy and colleagues reported that rapid pleurodesis could be achieved safely and effectively (successful rate: 92%) by combining thoracoscopy and talc poudrage with simultaneous tunneled pleural catheter in patients with symptomatic MPE (11). We did not use tunnelled catheter and thoracoscopy and we utilized talc-slurry which has been proved to be equally effective (12). In our study, 80.6% of patients could be treated less than 24 hours. This means that, rapid pleurodesis can lead to fast palliation without the need for hospitalization (i.e., in an outpatient setting) in most patients. Since the rapid pleurodesis is possible without a need for general anesthesia with a high success rate, we did not perform videothoracoscopic talc implementation in any of our patients.
There are a number of limitations in this study. Firstly, patients with small loculations were not treated in a specific manner (i.e., not tailored). We did not carry out cost analysis. The number of patients is relatively low. We aimed a total of 80 patients. Despite the fact that we analyzed 79 patients (not 80 patients as the statistical analysis suggested), the primary (the safety and effectiveness of rapid-pleurodesis) goal has been reached in the study. We also did not record and analyze the quality of life of the patients. However, it is plausible to speculate that, rapid pleurodesis could have provided acceptable quality of life since it is effective and shortens hospital stay comparing the standard plerurodesis group.
The findings of the current study showed that the new recommended method of 8 h aspiration intervals and immediate implementation of talc-slurry resulted in reduced days of drainage and hospitalization days (it can be done in outpatient basis) rendering no more morbidity than the standard procedure. Prolonged drainage and hospitalization seems unnecessary if the fluid is completely drained and the lung is re-expanded. Rapid and effective drainage and pleurodesis may lead to better comfort and prognosis in these usually low-performance patients. Further studies are needed to confirm this finding obtained by this rapid pleurodesis method.
Acknowledgements
Authors’ contributions: Serkan Özkul carried out the trial, Akif Turna designed and carried out the trial, Ahmet Demirkaya collected and analyzed data with Serkan Özkul and Burcu Aksoy. Burcu Aksoy collected and analyzed with Serkan Özkul and Ahmet Demirkaya. Kamil Kaynak supervised the study.
Disclosure: The authors declare no conflict of interest.
References
- Belani CP, Pajeau TS, Bennett CL. Treating malignant pleural effusions cost consciously. Chest 1998;113:78S-85S. [PubMed]
- Putnam JB Jr. Malignant pleural effusions. Surg Clin North Am 2002;82:867-83. [PubMed]
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001;18:402-19. [PubMed]
- Antunes G, Neville E, Duffy J, et al. BTS guidelines for the management of malignant pleural effusions. Thorax 2003;58:ii29-38. [PubMed]
- Sahn SA. Malignant pleural effusions. In: Shields TW, Locicero J, Reed CE, et al. eds. General Thoracic Surgery. 7th edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:875-83.
- Rodriguez-Panadero F, Antony VB. Pleurodesis: state of the art. Eur Respir J 1997;10:1648-54. [PubMed]
- West SD, Davies RJ, Lee YC. Pleurodesis for malignant pleural effusions: current controversies and variations in practices. Curr Opin Pulm Med 2004;10:305-10. [PubMed]
- Spiegler PA, Hurewitz AN, Groth ML. Rapid pleurodesis for malignant pleural effusions. Chest 2003;123:1895-8. [PubMed]
- Yildirim E, Dural K, Yazkan R, et al. Rapid pleurodesis in symptomatic malignant pleural effusion. Eur J Cardiothorac Surg 2005;27:19-22. [PubMed]
- Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet 2007;369:1535-9. [PubMed]
- Reddy C, Ernst A, Lamb C, et al. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011;139:1419-23. [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [PubMed]