Chemotherapy with paclitaxel plus carboplatin for relapsed advanced thymic carcinoma
Introduction
Thymic carcinoma is a rare and invasive mediastinal neoplasm (1). The role of systemic chemotherapy is important for advanced thymic carcinoma (2). For rarity of this tumor, no definitive chemotherapeutic regimen has been established. Several reports have indicated a well efficacy of different regimens, such as cisplatin + vincristine + doxorubicin + etoposide (CODE), cisplatin + doxorubicin + vincristine + cyclophosphamide (ADOC), and etoposide + ifosfamide + cisplatin (VIP) against thymic carcinoma in first-line treatment (3-5).
Paclitaxel has a mechanism of action that differs from older agents and the relatively less toxic profile of combination with platinum enables it to be used most widely in the treatment of many solid tumors (6). For the same epithelial origin of thymic tumors with other solid tumors, several studies detected the efficacy of paclitaxel plus carboplatin in thymic carcinoma, and some patients yielded a well response in first-line treatment (7,8). However, the efficacy data evaluation of paclitaxel plus carboplatin in second- or further-line treatment of thymic carcinoma is lacking.
We conducted a retrospective study to evaluate the efficacy and safety of paclitaxel plus carboplatin against advanced thymic carcinoma as second- or further-line chemotherapy.
Materials and methods
Patient eligibility
This retrospective study was conducted through a review of medical records of patients with advanced thymic carcinoma who received paclitaxel plus carboplatin as second- or further-line treatment between 2005 and 2012. Inclusion criteria were: (I) histological or cytological diagnosis of thymic carcinoma according to the histopathological criteria proposed by the WHO 2004 version; (II) Masaoka criteria stage IVa or IVb; (III) all patients received prior chemotherapy regimens; (IV) without any local treatment such as radiotherapy or interventional therapy during the paclitaxel plus carboplatin therapy time; (V) the recurrence or metastases performed with chest CT scans, abdominal, brain CT and bone scans.
Treatment methods
Paclitaxel was administered at a dose of 175 mg/m2 intravenously over 1 h, with the treatment cycle repeated every 3 weeks. Carboplatin (AUC =5) on day 1 was administered synchronous.
Responses and toxicity
Tumor responses were assessed every two cycles, or were evaluated early when significant signs of progression appeared. Objective tumor responses were according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Objective tumor responses included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The disease control rate (DCR) was defined as the addition of objective response and stabilization rates (CR + PR + SD). Toxicities were evaluated according to the National Cancer Institute Common Toxicity Criteria version 3.0 (CTC3.0).
Follow-up
All the patients that were evaluated for tumor response had a progression-free survival (PFS). Follow-up rate was 100%. Overall survival (OS) was defined as the time from the first day of diagnosis to death or last follow-up. PFS encompassed the time from the first cycle of paclitaxel plus carboplatin treatment to documented progression or death from any cause. The median follow-up period was 25.0 months (range, 5.5-65 months). The last follow-up time was Jan 31, 2014.
Statistical analysis
The survival curves were calculated according to the method of Kaplan-Meier. Statistical analysis was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of the treatment patients
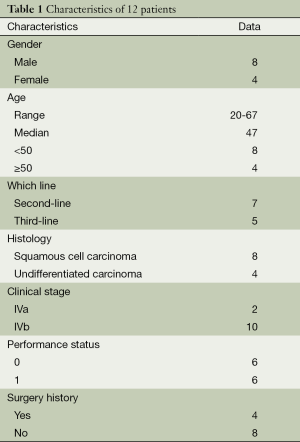
A total of 12 patients were included in the study. Among the 12 patients, resection was performed in four cases (three with median sternotomy and one with anterolateral thoracotomy). The first-line regimens of all the 12 patients contained CAP (cyclophosphamide + doxorubicin + cisplatin, n=7), VIP (n=3) and EP (etoposide+ cisplatin, n=2). Seven patients received paclitaxel plus carboplatin regimen as second-line and five as third-line chemotherapy. The median (range) number of paclitaxel plus carboplatin chemotherapy cycles was 3 (range, 1-4). Patients’ characteristics are shown in Table 1.
Full table
Response data and survival analysis
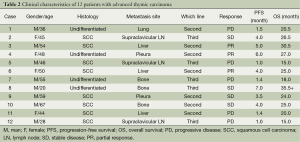
Response data for paclitaxel plus carboplatin therapy are shown in Table 2. Among the 12 patients, PR was confirmed in 3 patients and 4 showed SR, representing a response rate of 25.0% and DCR of 58.3%. There was no significant difference between the response (PR + CR) and no-response (SD + PD) patients among the gender (P=0.89), age (P=0.74), pathological subtype (P=0.81) and metastatic sites (P=1.0).
Full table
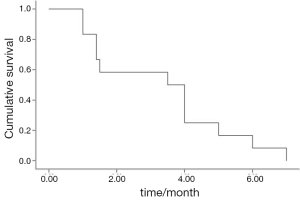
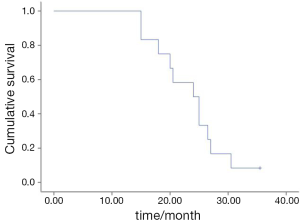
The median PFS was 3.5 months (95% CI, 1.4-5.6) (Figure 1). The median OS was 24.0 months (95% CI, 8.9-29.1) (Figure 2). There was no significant association for the PFS among the gender (P=0.68), age (P=0.21), stage (P=0.64) and pathological subtype (P=0.68). No difference was found in PFS between the second- and further-line chemotherapy (4.0 vs. 3.5 months, P=0.86).
Toxicity evaluation
All patients were assessed for toxicity. Grade 3/4 hematological toxicities were observed in three patients (one anemia, two neutropenia), and grade 3/4 non-hematological toxicities were seen in four patients (one fatigue, one hepatic injure, one neurotoxicity, one nausea). Two patients reduced the dosage for the grade 4 toxicity (one anemia and one nausea). No febrile neutropenia or toxic death occurred.
Discussion
To the best of our knowledge, our represents the largest data to assess whether paclitaxel plus carboplatin as second- or further-line chemotherapy confers any clinical benefit in patients with advanced thymic carcinoma. The outcome showed that paclitaxel plus carboplatin was useful as an alternative chemotherapy regimen for advanced thymic carcinoma in second or later-line treatment.
Standard systemic chemotherapy for advanced thymic carcinoma has not been determined because of its rarity. Anthracycline-based regimens such as CAP and ADOC were widely used in clinic. However, the toxicity of anthracycline-based regimens is a big question in clinical practice. Platinum-based chemotherapy such as TC (paclitaxel plus carboplatin) and EP is another choice for advanced thymic carcinoma and showed a well efficacy in several reports. A phase II study included 19 patients with thymic carcinoma showed a promising result with carboplatin and paclitaxel regimen as first-line chemotherapy (7). Followed this report, several retrospective studies have shown that carboplatin and paclitaxel regimen had a well efficacy and acceptable toxicity (7,8). Previous case series of second-line chemotherapy for advanced thymic carcinoma were based on regimens that were originally used for advanced thymoma or other solid tumors. Published case reports and series include treatment with S-1, irinotecan and docetaxel (9-11) (Table 3). The median PFS was ranged from 4 to 18 months. Only two studies totally included 6 patients showed a well efficacy of paclitaxel plus carboplatin regimen for advanced thymic carcinoma as second-line therapy (13,15). Our analysis indicated that the regimen of paclitaxel plus carboplatin was active for advanced thymic carcinoma patients as salvage chemotherapy with a response rate of 25.0% and median PFS of 3.5 months. The adverse events occurred at a similar incidence as previous reports in other solid tumors (6). Neutropenia is the most frequently reported ones and no patients refused further treatment for the toxicity.
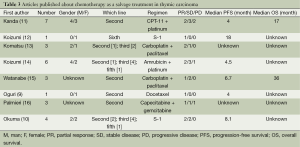
Full table
The major limitation of the present study is its retrospective nature and small number of patients. However, with no cases in prospective clinical studies, our retrospective study can also be considered to be meaningful.
Our results suggest that paclitaxel plus carboplatin is a promising regimen as salvage chemotherapy for pretreated advanced thymic carcinoma, but further studies are required to fully quantify the efficacy of this regimen.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Gubens MA. Treatment updates in advanced thymoma and thymic carcinoma. Curr Treat Options Oncol 2012;13:527-34. [PubMed]
- Wei ML, Kang D, Gu L, et al. Chemotherapy for thymic carcinoma and advanced thymoma in adults. Cochrane Database Syst Rev 2013;8:CD008588. [PubMed]
- Rea F, Marulli G, Di Chiara F, et al. Multidisciplinary approach for advanced stage thymic tumors: long-term outcome. Lung Cancer 2011;72:68-72. [PubMed]
- Loehrer PJ Sr, Jiroutek M, Aisner S, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma: an intergroup trial. Cancer 2001;91:2010-5. [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase-II trial of dose-dense chemotherapy in patients with disseminated thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9605). Br J Cancer 2009;101:1549-54. [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [PubMed]
- Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060-5. [PubMed]
- Igawa S, Murakami H, Takahashi T, et al. Efficacy of chemotherapy with carboplatin and paclitaxel for unresectable thymic carcinoma. Lung Cancer 2010;67:194-7. [PubMed]
- Oguri T, Achiwa H, Kato D, et al. Efficacy of docetaxel as a second-line chemotherapy for thymic carcinoma. Chemotherapy 2004;50:279-82. [PubMed]
- Okuma Y, Shimokawa T, Takagi Y, et al. S-1 is an active anticancer agent for advanced thymic carcinoma. Lung Cancer 2010;70:357-63. [PubMed]
- Kanda S, Koizumi T, Komatsu Y, et al. Second-line chemotherapy of platinum compound plus CPT-11 following ADOC chemotherapy in advanced thymic carcinoma: analysis of seven cases. Anticancer Res 2007;27:3005-8. [PubMed]
- Koizumi T, Agatsuma T, Komatsu Y, et al. Successful S-1 monotherapy for chemorefractory thymic carcinoma. Anticancer Res 2011;31:299-301. [PubMed]
- Komatsu Y, Koizumi T, Tanabe T, et al. Salvage chemotherapy with carboplatin and paclitaxel for cisplatin-resistant thymic carcinoma—three cases. Anticancer Res 2006;26:4851-5. [PubMed]
- Koizumi T, Agatsuma T, Ichiyama T, et al. Salvage chemotherapy with amrubicin and platinum for relapsed thymic carcinoma: experience in six cases. Med Oncol 2010;27:392-6. [PubMed]
- Watanabe K, Shinkai M, Goto H, et al. Chemotherapy with carboplatin and paclitaxel after failure of primary chemotherapy for advanced thymic carcinoma. A report of three cases and review of the literature. Tumori 2013;99:e172-6. [PubMed]
- Palmieri G, Merola G, Federico P, et al. Preliminary results of phase II study of capecitabine and gemcitabine (CAP-GEM) in patients with metastatic pretreated thymic epithelial tumors (TETs). Ann Oncol 2010;21:1168-72. [PubMed]