
Novel treatment strategies for early-stage lung cancer: the oncologist’s perspective
Introduction
Non-small cell lung cancer (NSCLC) is the most common type of lung tumors, and one of the leading causes of cancer deaths. The estimated number of new NSCLC cases in the United States (US) for year 2019 was 228,150, with an expected 142,670 deaths (1). Survival of patients with NSCLC dramatically differs according to disease stage at diagnosis. Recently, the updated 8th edition of NSCLC staging system has been released by the American Joint Committee for Cancer (AJCC), with significant revisions regarding both tumor (T) and node (N) descriptors (2). The lung cancer staging system was revised and validated by the International Association for the study of Lung Cancer (IASLC), and subsequently adopted by the AJCC (3-4). Early-stage disease, including stage I and stage II with negative nodes (N0), accounts for only 19% of NSCLC at diagnosis, whereas locally advanced [including stage II with nodal involvement (N+) and stage III] and metastatic disease, account for 24% and 55% of new cases, respectively (2). The 5-year overall survival (OS) rates range from 60% for localized disease, 33% for regionally spread disease, and drops down to 5.5% for subjects with distant metastases (5).
Multimodal approach, including surgery, radiotherapy and medical treatment, either alone or in combination (depending on disease status), can provide successful outcomes for early-stage disease. The mainstay of treatment for stage I and II tumors is radical surgery, which provides the best chance to cure (6). Patients who are deemed medically inoperable [unresectable stage I or stage II (T1–3 N0)] and/or refuse surgery, can be treated with definitive radiotherapy (7). Survival outcomes with radiotherapy is somehow similar to those obtained with surgery, with 5-year OS around 55% across different case series, even though with higher rates of locoregional recurrences (8). Post-operative radiotherapy can be considered in patients at high risk of recurrence (e.g., primary tumor >4 cm, positive margins), or in early-stage patients who are intraoperatively upstaged to N2 (9). Definitive chemoradiation is the preferred treatment choice for patients with stage II and III disease, who are not amenable with surgery.
Multidisciplinary management of patients from diagnosis is paramount, in order to grant the best treatment plan. However, unlike advanced/metastatic disease, little progress has been made in the treatment of early-stage NSCLC over the past decade. In the last years, efforts have been made to define the best treatment strategy according to disease sub-stage, to identify those patients who benefit from local intervention alone, not needing further medical treatment, and to explore novel treatment strategies. In this review we describe the standard treatment strategy according to disease stage, along with the results of the main clinical trials which have led to the recommended treatments. We also provide preliminary results of ongoing clinical trials exploring new treatment approaches for early-stage NSCLC, and give an overview of new treatment proposals that are currently under investigation in this setting.
Standard treatment approach
Adjuvant chemotherapy
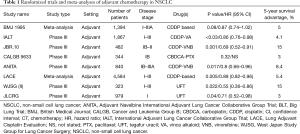
Adjuvant chemotherapy has demonstrated to provide a benefit in disease-free survival (DFS) and OS in early-stage NSCLC, with an absolute survival benefit of 4–5% compared to observation or best supportive care (10). Standard treatment consists of 4 courses of two-drug combination regimens platinum-based, plus vinorelbine, or other agents as paclitaxel, docetaxel, gemcitabine or pemetrexed. However, most of the evidences in NSCLC adjuvant chemotherapy trials is for the combination of cisplatin plus vinorelbine. Four large randomized trials and several smaller trials evaluated the role of cisplatin-based doublets (mainly cisplatin-vinorelbine), confirming a consistent benefit across stages II–IIIA after radical resection (11-14). Another trial evaluating the role of adjuvant carboplatin plus paclitaxel in stage IB resected patients, failed to demonstrate a survival advantage in this subgroup of patients (12). However, a statistically significant advantage was observed in patients who had primary tumors ≥4 cm, suggesting that adjuvant treatment should be considered as a treatment option in selected high-risk stage IB patients (12). Similarly, also a post-hoc exploratory analysis of the North American Intergroup phase III trial of adjuvant cisplatin plus vinorelbine (JBR-10 trial), showed that stage IB patients with tumors ≥4 cm appear to derive a clinically meaningful benefit from adjuvant chemotherapy (13). The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis evaluated the role of adjuvant chemotherapy in more than 4,500 NSCLC resected patients treated within the largest trials of adjuvant cisplatin-based chemotherapy. This meta-analysis confirmed an increased survival from 64% to 67% for stage IB, from 39% to 49% for stage II and from 26% to 39% for stage III NSCLC (10). Besides, a subgroup analysis of the LACE meta-analysis found that disease stage is a significant predictor for survival, with a 14.7% 5-year survival benefit for stage III, 11.6% for stage II, and 1.8% for stage I NSCLC (14). Taken together, these results suggest not only that patients with stage IA disease do not benefit from adjuvant chemotherapy but, most importantly, that adjuvant systemic treatment might be detrimental in this subset of patients (10). However, results of a meta-analysis of Japanese adjuvant chemotherapy trials with tegafur-uracil (UFT), showed that this treatment was associated with improved 5- and 7-year survival in a Japanese patient population composed primarily of stage I NSCLC patients (15). Table 1 displays information regarding the most relevant clinical trials and meta-analyses of adjuvant treatment for NSCLC.
Full table
On the basis of the above-mentioned results, adjuvant platinum-based chemotherapy has become the standard recommended treatment for stage II–III radically resected NSCLC. The European Society for Medical Oncology (ESMO) guidelines for early-stage NSCLC also suggest to consider adjuvant treatment in stage IB patients with primary tumor ≥4 cm (16). Altogether, results from randomized trials and meta-analyses demonstrated a detrimental role of adjuvant chemotherapy in stage IA, which is therefore not recommended in this setting.
Neoadjuvant chemotherapy
Besides its undiscussed benefit on survival, systemic treatment may be difficult to be delivered in the postoperative setting due to patients’ comorbidities and potential late recoveries after surgery. This issue was demonstrated by the NATCH phase III trial, which compared surgery alone to neoadjuvant or adjuvant chemotherapy with carboplatin plus paclitaxel (17). In the cohort of patients receiving preoperative chemotherapy, 90% of subjects completed the pre-planned three cycles of treatment, compared with only 61% of patients in the postoperative arm. However, no significant difference in DFS was observed in the three treatment arms. The same results emerged from another phase III randomized trial, which confirmed that preoperative and perioperative chemotherapy can provide superimposable survival benefit, response rate and quality of life (18).
In addition to better tolerability, neoadjuvant chemotherapy conveys several advantages, as the possibility to reduce tumor size, thus increasing operability rate, and prevention of micro-metastatic spread. On the other hand, disadvantages include delays in surgical intervention due to treatment-related toxicity, and the possibility that the tumor is still considered unresectable after chemotherapy.
Several trials have assessed the benefit of neoadjuvant treatment compared with surgery alone. The phase III trial LU22/NALVT/EORTC, randomized more than 500 patients to receive surgery alone vs. neoadjuvant chemotherapy followed by surgery (19). This trial demonstrated that neoadjuvant treatment was feasible, did not negatively impact on the incidence of post-operative complications, and had a 49% response rate (95% CI: 43–55%). OS rates between the two arms, however, were similar [hazard ratio (HR): 1.02; 95% CI: 0.80–1.31; P=0.86]. The SWOG 9900 trial had a comparable design, and randomized 354 patients to receive surgery vs. neoadjuvant chemotherapy plus surgery (20). The median OS in the neoadjuvant plus surgery arm was better than the surgery alone arm (62 vs. 41 months, respectively). However, this trial closed early since results from ongoing trials demonstrated higher survival benefit from adjuvant therapy. Also, the chemotherapy in early-stages NSCLC trial (ChEST), comparing neoadjuvant gemcitabine/cisplatin followed by surgery with surgery alone, prematurely closed, after recruiting fewer than half of the 700 pre-planned patients. Results from this trial, however, showed that HRs for both PFS and OS favored neoadjuvant chemotherapy followed by surgery HR for PFS: 0.70 (95% CI: 0.50–0.97; P=0.003), and HR for OS: 0.63 (95% CI: 0.43–0.92; P=0.02) (21).
The NSCLC Meta-analysis Collaborative Group provided results of a pooled analysis of 15 randomized trials of neoadjuvant chemotherapy plus surgery vs. surgery alone, including nearly 2,500 patients (22). Results from this meta-analysis suggest that neoadjuvant treatment provides a significant survival advantage through all patients’ subgroups (regardless of age, and disease stage), with a 13% reduction in the relative risk of death. Table 2 displays information regarding the most relevant clinical trials and meta-analyses of neoadjuvant treatment for NSCLC.

Full table
Overall, neoadjuvant treatment for early-stage NSCLC has not been evaluated as extensively as postoperative in randomized trials, however evidence suggest that both treatments might have the same efficacy on survival outcomes. Similarly, there are few head-to-head trials of adjuvant vs. neoadjuvant chemotherapy. The indirect comparison meta-analysis published by Lim et al., evaluated the relative HRs for survival of neo- and adjuvant chemotherapy trials. This meta-analysis included 22 trials of adjuvant treatment, and 10 trials of neoadjuvant treatment, for a total 10,000 patients (23). Relative HR for OS of adjuvant vs. neoadjuvant was 0.99 (95% CI: 0.81–1.21; P=0.91), while relative HR for DFS was similar in the two groups of studies. Therefore, the best timing of chemotherapy delivering remains unclear, however evidence suggest that this does not significantly impact on survival outcomes. In clinical practice, pre-operative chemotherapy might be considered in selected early-stage patients who might benefit from disease downstaging, potentially resulting in a less extensive resection.
Open questions and future perspectives
In recent years, most clinical trials in early-stage NSCLC has focused on moving effective therapies currently in use for metastatic disease (other than conventional cytotoxic chemotherapy), in earlier phases of treatment. The rationale of adjuvant therapy to eradicate the minimal residual disease, in order to reduce the risk of relapse, makes the possibility to use more precise drugs particularly appealing. Similarly, the possibility to use effective drugs in neoadjuvant setting is appealing, not only to increase resectability rate, but also to have information on pathological response to treatment at the time of surgery (see further). Here, we will provide the most important evidence to date and the main ongoing clinical trials of novel drugs in adjuvant and neoadjuvant setting.
Adjuvant treatment
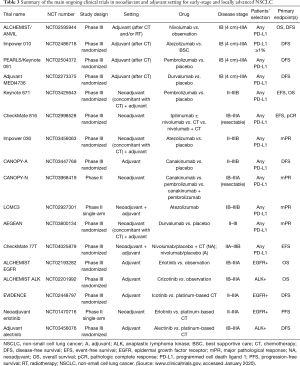
One of the most important class of drugs to be explored in the adjuvant setting are tyrosine kinase inhibitors (TKIs) targeting oncogenic drivers’ mutations, mostly epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) inhibitors. Preliminary results from a randomized trial of adjuvant cisplatin-vinorelbine versus the TKI gefitinib in EGFR positive NSCLC, suggest that this treatment might lead to better DFS; however, OS data for this trial are not mature yet (24). The open-label phase II SELECT trial evaluated the role of 2 years of erlotinib treatment after adjuvant chemotherapy (with or without radiotherapy), showing an improvement in DFS for patients treated with erlotinib (25). Prospective trials of adjuvant targeted therapies in patients with radically resected NSCLC harboring oncogenic drivers are presently ongoing (26-30) (Table 3). Results from these trials will help to clarify the potential role of TKIs in the adjuvant setting, either as exclusive treatment or as a maintenance after cytotoxic chemotherapy. According to the available results to date, there is probably a subgroup of patients who can benefit from the use of adjuvant TKIs, given the increased DFS, major tolerability and improved quality of life. However, there is still limited evidence on the role of adjuvant TKIs in oncogene addicted NSCLC (31), therefore their use in the adjuvant setting is not recommended outside clinical trials.
Full table
The second most promising class of drugs is represented by immune checkpoint inhibitors (ICIs). NSCLC is an immunogenic tumor, and immunotherapy has demonstrated a significant clinical activity with 15–20% durable responses in advanced/metastatic disease (32,33). The most widely studied ICIs in this setting are the monoclonal antibodies targeting the anti-programmed cell death 1 (PD-1), nivolumab, and pembrolizumab; the PD-1 ligand (PD-L1), atezolizumab, and durvalumab; and the cytotoxic T-lymphocyte antigen-4 (CTLA-4), ipilimumab. Immunotherapy has been explored both in the adjuvant and neoadjuvant setting for early-stage NSCLC. Evidence from cutaneous melanoma suggest that ICIs might be effective in the setting of minimal residual disease, in which the tumor microenvironment is immature and therefore easier to overcome, compared to metastatic disease (34).
Several trials of adjuvant anti-PD-1/PD-L1 in resected stage IB-IIIA NSCLC are ongoing, all of them investigating the use of immunotherapy as a maintenance after adjuvant chemotherapy, either alone or combined with radiotherapy (35,36) (Table 3). Results from this trial are not available yet, and will probably help to solve some open questions: adjuvant immunotherapy might be influenced by previous treatments (i.e., surgery, radiotherapy, and chemotherapy), which act on immune cells stimulating an immune-suppressive or an immunogenic microenvironment. Moreover, the subset of patients who can benefit more from adjuvant immunotherapy is yet to be identified, specifically concerning disease stage and PD-L1 expression. Thus, patients’ selection, both regarding disease characteristics and previous treatment, might become an important issue to select the most appropriate adjuvant treatment.
Neoadjuvant treatment
Immunotherapy for treatment of early-stage NSCLC is even more promising in the neoadjuvant setting. The biologic rationale lies in the possibility to turn the in-place tumor into an “auto-vaccine”, thereby inducing anti-tumor immune responses that act through the body against micro-metastases, thus reducing the risk of disease relapse. More importantly, preoperative systemic treatment can give information regarding pathological response on resected tumor at the time of surgery. In the near future, this might potentially translate in the chance to customize further adjuvant treatment, defining it on the basis of major pathological response obtained with preoperative treatment. A pilot study of neoadjuvant nivolumab showed that 45% of patients with surgically resectable stage I–IIIA NSCLC reach major pathological response with only 2 courses of preoperative nivolumab, with few treatment-related side effects and without surgery delay (37). Combination therapy with nivolumab and ipilimumab in the NEOSTAR phase II trial has induced 26% major pathological response and changes in immune infiltrates of stage I–IIIA resectable NSCLC (38). The combination of preoperative nivolumab plus chemotherapy (carboplatin-paclitaxel) in patients with resectable stage IIIA NSCLC was tested in the phase II NADIM trial, with 80% of patients reaching major pathological response (39). The LCMC3 phase II trial is currently evaluating neoadjuvant atezolizumab in patients with resectable early-stage (IB–IIIB) NSCLC. Preliminary results from an initial safety analysis of the first 54 of 180 planned patients was described: 16 patients had grade 3 or 4 adverse events (AEs) (three of them were considered treatment-related); surgery was delayed in one subject because of grade 3 pneumonitis. Major pathological response rate was 24%, and 58% (11/19) of patients had less than 50% viable tumor on surgical specimen (40). Table 2 summarizes the principal studies of adjuvant and neoadjuvant ICIs for early-stage NSCLC with preliminary results and trials currently ongoing.
At least two major considerations should be made on preliminary results of innovative treatments in adjuvant and neoadjuvant treatment of early-stage NSCLC. First, these results come from small populations of selected patients: everyday clinical practice might not always mirror that of clinical trials, not only for what regards patients’ characteristics, but also regarding the feasibility of a specific multimodal treatment. Moreover, clinical trials presented to date widely differ in type of adjuvant treatment, and the rationale for the choice of an adjuvant specific systemic treatment on the basis of major pathological response should be defined. Lastly, treatment related AEs during immunotherapy, and specifically with combined ICIs, is a matter of concern in patients receiving treatment for metastatic disease. An accurate estimate of risks to benefit ratio should be made considering the early-stage of disease and the adjuvant setting.
Conclusions
Radical surgical resection followed by observation remains the best treatment strategy for early-stage NSCLC. Adjuvant chemotherapy with cisplatin-based doublets provide an advantage in survival for radically resected stage IB–IIIA and is currently the standard treatment. The future of adjuvant and neoadjuvant treatment in early-stages involve combination treatment with targeted agents and ICIs, and several trials are currently underway with preliminary promising results. Customization of treatment on patients’ characteristics before, and major pathological response after therapy, will further improve survival outcomes in this subset of patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mario Nosotti, Ilaria Righi and Lorenzo Rosso) for the series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.46). The series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts and Figures 2019. Atlant: American Cancer Society, 2019.
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. Hoboken: John Wiley & Sons, Inc., 2017.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eight) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2017;12:1109-21.
- National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975-2016. Available online: https://seer.cancer.gov/csr/1975_2016/
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- van den Berg LL, Klinkenberg TJ, Groen HJ, Widder J. Patterns of Recurrence and Survival after Surgery or Stereotactic Radiotherapy for Early Stage NSCLC. J Thorac Oncol 2015;10:826-31. [Crossref] [PubMed]
- Burdett S, Stewart L, Group PM. Postoperative radiotherapy in non-small cell lung cancer: update of an individual patient data meta-analysis. Lung Cancer 2005;47:81-3. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV. LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29-34. [Crossref] [PubMed]
- Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol 2010;5:220-8. [Crossref] [PubMed]
- Hamada C, Tanaka F, Ohta M, et al. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol 2005;23:4999-5006. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-21. [Crossref]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Westeel V, Quoix E, Puyraveau M, et al. A randomized trial comparing preoperative to perioperative chemotherapy in early-stage non-small-cell lung cancer (IFCT 0002 trial). Eur J Cancer 2013;49:2654-64. [Crossref] [PubMed]
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRCLU22/NVALT 2/EORTC08012 multicentre randomised trial and up-date of systematic review. Lancet 2007;369:1929-37. [Crossref] [PubMed]
- Ball D, Mitchell A, Giroux D, et al. Effect of tumor size on prognosis in patients treated with radical radiotherapy or chemoradiotherapy for non-small cell lung cancer. An analysis of the staging project database of the International Association for the Study of Lung Cancer. J Thorac Oncol 2013;8:315-21. [Crossref] [PubMed]
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [Crossref] [PubMed]
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Lim E, Harris G, Patel A, et al. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380-8. [Crossref] [PubMed]
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomized, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. [Crossref] [PubMed]
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: a phase II trial of adjuvat erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol 2019;37:97-104. [Crossref] [PubMed]
- Tada H, Takeda K, Nakagawa K, et al. Vinorelbine plus cisplatin versus gefitinib in resected non-small cell lung cancer harboring activating EGFR mutation (WJOG6410L). J Clin Oncol 2012. [Crossref]
- National Cancer Institute. The ALCHEMIST lung cancer trials. Available online: http://www.cancer.gov/types/lung/research/alchemist
- Wu YL, Herbst RS, Mann H, et al. ADAURA: phase III, double-blind, randomized study of osimertinib versus placebo in EGFR mutation-positive early-stage NSCLC after complete surgical resection. Clin Lung Cancer 2018;19:e533-6. [Crossref] [PubMed]
- Shen P, Zhong W. Adjuvant EGFR TKI therapy for resectable non-small cell lung cancer: new era for personalized medicine. J Thorac Dis 2018;10:1364-9. [Crossref] [PubMed]
- Govindan R, Mandrekar SJ, Gerber DE, et al. ALCHEMIST trials: a golden opportunity to transform outcomes in early stage non-small cell lung cancer. Clin Cancer Res 2015;21:5439-44. [Crossref] [PubMed]
- Huang Q, Li J, Sun Y, et al. Efficacy of EGFR tyrosine kinase inhibitors in the adjuvant treatment for operable non-small cell lung cancer by a meta-analysis. Chest 2016;149:1384-92. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Reck M, Rodrìguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Weber J, Mandalà M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824-35. [Crossref] [PubMed]
- Chaft JE, Dahlberg SE, Gerber DE, et al. EA5142 adjuvant nivolumab in resected lung cancers (ANVIL): The newest study in the ALCHEMIST platform. J Clin Oncol 2017. [Crossref]
- Hellyer J, Wakelee H. MS04. 03 Adjuvant PD-(L) 1 checkpoint inhibitors. J Thorac Oncol 2019;14:S156-7. [Crossref]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Cascone T, William WN, Weissferdt A, et al. LBA49 neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC). Ann Oncol 2018;29:mdy424.059.
- Provencio M, Nadal E, Insa A, et al. OA13.05 NADIM study: updated clinical research and outcomes. J Thorac Oncol 2019;14:S241. [Crossref]
- Blumenthal GM, Bunn PA Jr, Chaft JE, et al. Current status and future perspectives on neoadjuvant therapy in lung cancer. J Thorac Oncol 2018;13:1818-31. [Crossref] [PubMed]


