Optical imaging versus CT and plain radiography to quantify pectus severity: a systematic review and meta-analysis
Introduction
Pectus excavatum (PE) and pectus carinatum (PC) are the most common congenital chest wall deformities. The latter is characterized by an outward protrusion of the sternum, while PE is characterized by an inward depression. PE occurs in 1:400 of live births (1), in comparison to PC which is reported to occur 2–4 times less frequent (2). PE and PC may be associated with impaired body image perception and result in lowered self-esteem, psychological stress and diminished quality of life. Next to these psychological effects, PE may be associated with impaired cardiopulmonary function (3,4). The current gold standard to evaluate the extent of pectus deformities is computed tomography (CT). In patients with PE, CT is generally used to calculate the Haller index (HI) (5). The resulting HI is subsequently used in the process of decision making to determine surgical candidacy. In PC no such standard metric exists. Despite CT being the current gold standard, it inescapably implies exposure to ionizing radiation. Two-view chest radiographies may be used alternatively to CT, resulting in dose reduction. However, according to the doctrine of radiation hygiene, every effort should be made to avoid, or if not possible, limit radiation exposure, especially in pediatric patients with a long lifetime risk to develop radiation related pathologies (6,7). In an effort to diminish radiation exposure, alternative methods are being explored to quantify the extent of chest wall deformities, among which three-dimensional (3D) optical surface imaging shows great potential (8). This technology has been widely used to map the chest surface in scoliosis patients and may serve as a safe and non-invasive severity measurement tool that utilizes non-ionizing light illumination. Optical imaging produced trunk topographies have already been demonstrated to be clinically feasible and accurate (9,10). However, as stressed by Sarwar and colleagues (8), the exact clinical value (e.g., in the process of decision making, follow-up, et cetera) of this novel severity measurement technique in PE and PC is yet to be investigated. Consequently, the following research question was formulated: can the evaluation of pectus excavatum and carinatum severity through chest CTs and radiographies be replaced by 3D optical scans? To answer this question, we conducted a systematic review and pooled analysis of the currently available literature in which we assessed all studies that compared 3D optical scan-based severity measurements with those derived from CT-scans or chest radiographies in patients with PE and PC. To our knowledge no such comprehensive review has been conducted to date.
Methods
Protocol and registration
Prior to start, the review protocol was registered to the PROSPERO registry (Record ID: CRD42019122860). In addition, this review was written in compliance with the PRISMA statements to ensure quality and transparency throughout (11).
Eligibility criteria
Types of participants
Patients of any race, gender and age with PE or PC were considered for inclusion.
Types of intervention
Papers that performed pectus severity quantification based on 3D optical imaging and compared its performance to severity measurements based on chest radiographies or CT-scans were examined for eligibility. All optical surface imaging techniques, such as laser and structured (white) light, were considered for inclusion. Contact 3D scanners that probe the subject through physical touch were not considered.
Primary outcome measure(s)
Comparison of pectus severity measurements based on 3D optical surface scanning and the study’s comparative measurement method (i.e., CT-scans or chest radiographies).
Types of studies
All observational and randomized studies adhering to the aforementioned criteria were assessed for eligibility. Studies reporting combined data on PE and PC severity measurements were considered only if data were presented separately.
Search and study selection
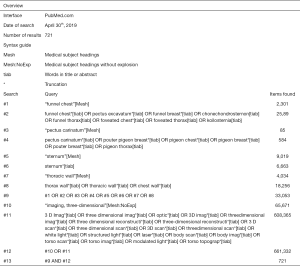
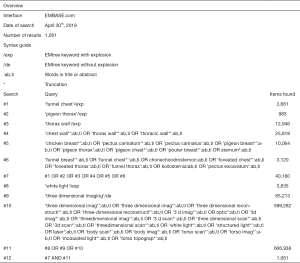
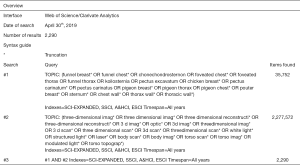
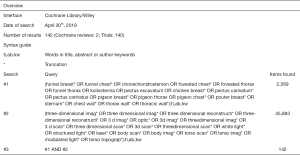
Potentially eligible papers were identified by searching electronic scientific databases and trial registries. Solely articles reported in English were considered. No publication date restrictions were imposed. The search strategy was first applied to the PubMed database and subsequently adapted for EMBASE, Web of Science, the Cochrane Library, and CINAHL. In addition, the PROSPERO, WHO-ICTRP, and Clinicaltrials.gov registries were searched. See Figures S1-S5 for the complete scientific database search queries. Identical queries were used to search the aforementioned registries. An additional manual cross-reference and related-articles search was conducted to identify articles that were not found through the prior search. This additional step also functioned as an indicator of the quality and integrity of the database search strategy. All searches were performed by a certified librarian. The last search was run on April 30th, 2019. Articles resulting from the searches were judged for eligibility based on their title and abstract. Thereafter, full text of potentially eligible articles was read and assessed according to the predefined eligibility criteria. Papers meeting these criteria were included for systematic review, and if applicable, meta-analysis. Article selection was performed in a standardized, unblinded manner by two independent reviewers (Jean H. T. Daemen & Tom G. J. Loonen). Inter-reviewer disagreements were resolved by consultation of Erik R. de Loos.
Data collection and data items
Data was extracted by one independent reviewer (Jean H. T. Daemen) and validated by a second reviewer (Tom G. J. Loonen). Inter-reviewer disagreements were resolved by consultation of Erik R. de Loos. To structure data extraction and presentation, an extraction sheet was developed and pilot-tested on two randomly selected included studies. The sheet was adopted accordingly. Data was extracted from each included paper on: (I) general study characteristics: study design, country and enrolment period; (II) characteristics of participants: number of included participants, gender, age, and the thoracic wall deformity that was studied (i.e., PE or PC); (III) characteristics of the optical scan(ner): scanner brand/type, scanning method, static or handheld, accuracy, acquisition and/or processing time, used software, patient position, and pectus severity measurement method; (IV) characteristics of the comparison: comparative technique (e.g., CT-scans or radiographies), and severity measurement method; (V) primary outcome measure: comparison of 3D optical surface scan- and radiography- or CT-based severity measurements. Continuous variables were denoted as mean, standard deviation (SD) and range. Continuous variables reported as median and interquartile range or standard error were converted. The primary outcome measure was extracted as reported. For studies that solely reported raw severity measurement data, Pearson’s correlation coefficients (r) were calculated using SPSS statistics (IBM Corp. Released 2017. IBM SPSS statistics for MacOS, version 25.0, Armonk, NY, USA). The Pearson’s correlation coefficient was chosen as this was the most used metric to compare severity indices as found during the preliminary search. Missing P values were, if possible, calculated from the available data. P≤0.05 was considered to be statistically significant. To interpret the size of reported correlation coefficients, we used cut-off values as described by Mukaka (12). Correlation sizes that ranged from 0 to 0.30 were judged to be negligible, while correlations that ranged from 0.30 to 0.50, 0.50 to 0.70, 0.70 to 0.90, and 0.90 to 1.00 were interpreted as either low, moderate, high and very high.
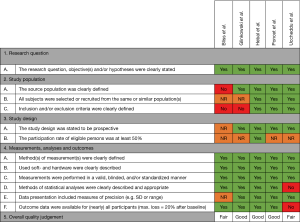
Risk of bias in individual studies
No validated tools exist that assess quality of correlation studies. Therefore, a tool was constructed (See Figure 1). This tool was adapted from the National Heart, Lung, and Blood Institute Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (13) and pilot tested on two randomly selected included studies. The tool was adopted accordingly. Questions were answered by Yes, No, not applicable (NA), or not reported (NR). Studies were, subsequently, given an overall quality judgement (Good, Fair, or Poor). This judgement was not based on simple summation of answers but based on the ability of studies to draw associative conclusions about the effect of the imaging techniques being studied on outcomes. Quality assessment was performed by two reviewers (Jean H. T. Daemen & Tom G. J. Loonen). Inter-reviewer disagreements were resolved by consultation of Erik R. de Loos.
Summary measures and synthesis of results
Quantitative synthesis of the primary outcome measure was only performed if studies were sufficiently homogeneous, otherwise, data was reported as such. For quantitative synthesis, correlation coefficients were converted into Z-scores using the Fisher Z-transformation method. Resulting Z-scores were pooled using the random-effects model. Pooled Z-scores and their corresponding 95% confidence interval (95% CI) were converted back into pooled correlation coefficients to allow easy interpretation. No additional analyses were performed. The I2-test for statistical heterogeneity was used as a measure of consistency. I2 values greater than 50%, with a P value ≤0.10 indicated the presence of substantial heterogeneity. Meta-analyses were performed by ProMeta 3.0 software for MacOS (based on ProMeta 2.1, deployed by Internovi, Cesena, Italy).
Risk of bias across studies
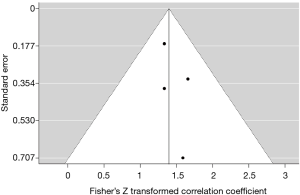
Publication bias was assessed both visually by a funnel plot (a standard error by Z-score plot of the primary outcome measure), and statistically with Egger’s linear regression, and Begg’s and Mazumdar’s rank correlation tests. A P value ≤0.10 was considered statistically significant. Publication bias analyses were performed by ProMeta 3.0 software for MacOS (based on ProMeta 2.1, deployed by Internovi, Cesena, Italy).
Results
Study selection
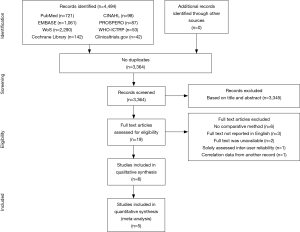
See flow diagram, Figure 2. The PubMed (n=721), EMBASE (n=1,061), Web of Science (n=2,290), Cochrane Library (n=142), CINAHL (n=98), PROSPERO (n=87), WHO-ICTRP (n=53), and Clinicaltrials.gov (n=42) databases and registries provided a total number of 4,494 citations. No citations were obtained through the related-articles and cross-reference searches. No unpublished data was obtained. Of the 4,494 citations, 1,130 duplicates were discarded using the Mendeley find duplicates function (Mendeley Desktop v1.19.4 for MacOS, Mendeley Ltd., Elsevier). An additional 3,345 studies were discarded because their title and/or abstract did not comply with the predetermined eligibility criteria. Full texts of the remaining 19 papers were read, whereupon another 14 papers were excluded for systematic review. Reasons for exclusion were: lack of a comparative method (n=6); the full text was NR in the English language (n=3); only a conference abstract was available (n=2); the fact that only inter-user reliability was assessed (n=1); PE and PC data were not presented separately (n=1) or the use of correlation data from another article (n=1). Eventually, 5 papers were considered eligible for systematic review and qualitative synthesis, while 4 papers were also included for quantitative synthesis (i.e., meta-analysis).
Study characteristics
Methods
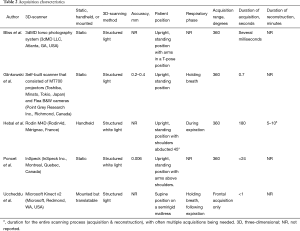
See Table 1. All included papers conducted an observational study, of which 4 were stated to be prospective (14-17). No randomized controlled trials were included. Studies were conducted in Canada, Italy, Poland, Spain or the USA, participants were enrolled between 2005 and 2017.

Full table
Participants
In total, 75 participants were enrolled. All studies included participants with PE whereas the study performed by Poncet et al. (16) was the only study to also include 5 participants with PC (Table 1). This study was not excluded because PE and PC data were reported separately. The percentage of male subjects with PE ranged from 80% to 100%, as reported by Bliss et al. (18) and Glinkowski et al. (14). Individual sample sizes ranged from 4 to 39 participants. The mean age of participants with PE was reported by Bliss et al. (18), Glinkowski et al. (14) and Poncet et al. (16) and ranged from 13.8 to 16.5 years. The mean age of participants with PC was 11.6 (SD: 4.5) years (16).
Intervention
3D thoracic surface scan
See Tables 2,3. All studies utilized 3D scanners of different manufacturers. Three studies used a static scanner type (14,16,18), while Hebal et al. (15) used a handheld scanner and Uccheddu et al. (17) mounted their scanner on a specially devised frame that allowed vertical translation. Despite these differences, all scanners used structured (white) light projectors to detect the 3D thoracic surface. Prior to acquisition, 4 studies (14-16,18) positioned their participants in an upright standing position with the arms at the level of, or above the shoulders. The same 4 studies all acquired a 360° thoracic surface scan. In contrast, Uccheddu et al. (17) positioned their participants in a supine position on a semi rigid mattress and solely obtained a frontal acquisition. Among the reported studies, scans were all acquired at different phases of the respiratory cycle (14,15,17). Acquisition of the 3D scans took several milliseconds to 180 seconds whereas reconstruction took up to 10 minutes (15,18). Scanner accuracy was reported to be 0.2–0.4 mm. for the study of Glinkowski et al. (14) and 0.006 mm. for Poncet et al. (16).
Full table
Full table
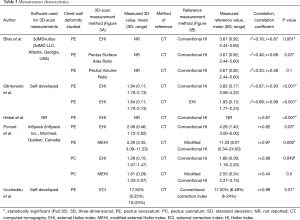
In four studies thoracic surface scan-based PE severity measurements were calculated by dividing the widest external thoracic transverse diameter by the distance between the external vertebral body and external deepest point (Figure 3A) (14-16,18). Terminology of this measure differed among all four studies but will from this point on be referred to as the external Haller index (EHI). Bliss et al. (18) additionally derived two, self-developed PE measures: The Pectus Volume Ratio and Surface Area Ratio. The latter was obtained by calculating the ratio between the surface area of both the chest deformity (i.e., the area beneath the normal aspect of the anterior chest) and torso (i.e., sternal notch to xiphoid). The same applies to the Volume Ratio, for which volumes were used. In addition, Poncet et al. (16) also reported another self-developed PE measure that was modified from the EHI; the modified external Haller index (MEHI) (Figure 3A). This measure was calculated by dividing the widest external thoracic transverse diameter by the anteroposterior distance from the imaginary transverse diameter line to the external deepest point. Uccheddu et al. (17) only calculated the external Correction index (ECI) (Figure 3A), that is defined as: (d–e)/d, where d and e are the vertical distances of, respectively, the minimum and maximum sternal depression with respect to the reference plane (i.e., the semirigid mattress plane).
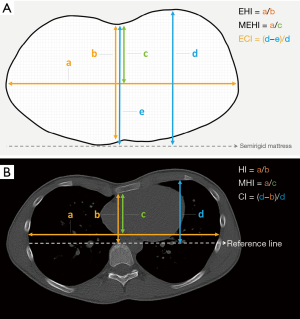
To determine the severity of PC, Poncet et al. (16) utilized similar measures as for PE, however, for PC the point of maximal protrusion was used as reference point.
Comparison
See Table 3. To assess 3D surface scan performance, all studies acquired a comparative thoracic CT-scan (14-18); the current gold standard for pectus severity quantification. All studies analyzed thoracic surface and CT-scan based measurements of the same participant. In comparison to the aforementioned 3D scan measures, CT-scan derived severity measurements were based on internal diameters. Four out of 5 studies calculated the conventional HI (Figure 3B) to determine PE severity (14-16,18). The HI was obtained by dividing the widest inner thoracic diameter by the anteroposterior distance from the posterior sternal surface to the anterior vertebral surface. Glinkowski et al. (14) additionally derived the CT-based EHI to assess 3D and CT-scan agreement. In line with the aforementioned thoracic surface scan indices, Poncet et al. (16) reported an additional, self-developed modified Haller index (MHI) (Figure 3B), obtained by dividing the widest internal transverse diameter by the anteroposterior distance from the imaginary widest internal transverse diameter line to the posterior sternal surface. In comparison, Uccheddu et al. (17) used the Correction index to determine the CT-based PE severity. This index measures the percentage of PE to be corrected and is calculated by the following formula (Figure 3B): (d–b)/d, where d and b are the vertical distances of, respectively, the minimum and maximal sternal depression with respect to the anterior vertebral body reference line.
To determine the CT-based PC severity, Poncet et al. (16) again utilized similar measures as for PE, however, for PC the point of maximal protrusion was used as point of reference.
Outcomes
One paper compared its thoracic surface and CT-scan derived pectus severity measurements using the Pearson’s correlation coefficient (r) (15) while two studies reported the squared variant (r2) (14,18). Uccheddu et al. (17) reported the raw outcome data only, whereas Poncet et al. (16) reported correlation data for their entire study population (i.e., PE and PC combined) including the raw data. The level of statistical significance was only denoted by Hebal et al. (15). Missing correlation coefficients and P values were calculated post hoc.
Risk of bias within studies
See Figure 1 for the risk of bias assessment per study. The study of Glinkowski et al. (14), Hebal et al. (15) and Poncet et al. (16) were all judged to be of good methodological quality; i.e., outcome measures were not doubted. The study of Bliss et al. (18) and Uccheddu et al. (17) were considered to be of fair methodological quality.
Synthesis of results
Qualitative synthesis
See Table 3. For PE, correlation sizes (r) ranged from 0.87 to 0.93 among studies that assessed the correlation of 3D scan derived EHI and CT-scan derived HI (14-16,18). These correlations were all statistically significant. The size of correlation was not affected by the type of 3D scanner used, although the use of a handheld scanner was associated with a prolonged acquisition time. Bliss et al. (18) additionally determined their self-developed 3D scan based Pectus Surface Area Ratio and Pectus Volume Ratio and assessed its agreement to the conventional CT-based HI. They found moderate positive correlations of 0.68 (P=0.03) and 0.58 (P=0.1), respectively. In a similar manner, Glinkowski et al. (14) assessed the correlation of the 3D- and CT-scan derived EHI and found a coefficient of 0.99 (P<0.001); suggesting near perfect agreement between both acquisitions. Poncet et al. (16) additionally assessed the correlation of their self-constructed MHI and MEHI that was respectively obtained from acquired CT- and 3D-scans of participants with PE. Correlation of these modified indices (r=0.98; P<0.001) was slightly superior to the correlation of the 3D-EHI and CT-scan derived HI (r=0.96; P<0.001). However, superiority of the 3D scan derived MEHI over the EHI remains unknown, while the MEHI was not compared to the gold standard (i.e., CT derived HI). Uccheddu et al. (17) quantified PE severity utilizing the 3D-derived ECI and CT-scan derived CI, and found a correlation coefficient of 0.99 (P=0.01).
As mentioned in the previous sections, Poncet et al. (16) also investigated the correlation of the 3D scan derived EHI and MEHI with the CT-based conventional HI and MHI to determine the severity of PC. They found a high and low correlation of 0.88 (P=0.049) and 0.44 (P=0.5), respectively.
Quantitative synthesis
The study of Bliss et al. (18), Glinkowski et al. (14), Hebal et al. (15) and Poncet et al. (16) were found to be sufficiently homogenous to be admitted for quantitative synthesis because they all included patients with PE and utilized identical severity metrics. From the study of Poncet et al. (16), only participants with PE were included for quantitative synthesis. Inspection of the individual correlation coefficients and forest plot (Figure 4) indicated the presence of an overall high positive correlation between the CT-based HI and 3D scan-based EHI. This was statistically confirmed by meta-analysis (Figure 4) that demonstrated a pooled Z-score of 1.40 (95% CI: 1.13 to 1.66; P<0.001) These Z-scores corresponded with a pooled correlation coefficient of 0.89 (95% CI: 0.81 to 0.93; P<0.001). No heterogeneity was detected (I2=0.00%; P=0.834). No subgroup analyses were performed.

Risk of bias across studies
A funnel plot of the studies that were included for quantitative synthesis was constructed. Graphical assessment demonstrated no evident asymmetry; indicating the absence of publication bias (Figure 5). This was statistically reproduced by Begg’s and Mazumdar’s rank correlation test (P=0.497) and by Egger’s linear regression test (P=0.407).
Discussion
This systematic review and meta-analysis examined all studies that compared the use of 3D optical imaging and CT-scans or chest radiographies in the quantification of pectus severity. Based on the previously described eligibility criteria, 5 observational studies were included, enrolling a total of 75 participants. Of these, 70 were participants with PE. No studies were judged to be of poor methodological quality. No studies were included that assessed the use of two-view plain radiographies. All studies utilized CT-scan based severity metrics as comparison; the current gold standard for severity quantification. To assess 3D- and CT-scan agreement, all studies calculated correlation coefficients or correlation coefficients could be determined from the available raw data. Only one of these studies investigated the correlation among participants with PC (16). This low number of studies describing the use of 3D scans to determine PC severity may be a direct consequence of the absence of a standardized PC severity measure. Among participants with PC (n=5), Poncet et al. (16) found a high correlation of 0.88 between the CT-based HI and 3D-based EHI with a 95% CI that ranged from –0.01 to 0.92. We subsequently concluded that with the currently available limited data no evidence can be produced to either support nor discard the use of 3D scans to determine PC severity in comparison to CT-scans and chest radiographies. Nevertheless, 3D scans may be used to monitor treatments such as compressive orthotic bracing as described by Wong et al. (19).
Four out of five included studies acquired identical 3D scan and CT derived PE severity indices and were subjected to meta-analysis. Pooled analysis revealed a high positive, statistically significant correlation between the optical scan measured EHI and CT-scan derived conventional HI (r=0.89; P<0.001). Although pooled analysis demonstrated a high correlation, 3D thoracic surface scan derived External Haller indices are not yet a valid tool to aid in the multifactorial process of surgical decision making. Correlation coefficients express the direction and magnitude of a linear relationship between two measures, but they do not assess their exact agreement. This is best illustrated by Glinkowski et al. (14) and Poncet et al. (16) who found mean 3D scan-based EHI values of 1.84 and 1.67, with corresponding mean CT-based HI values of 3.82 and 2.97. Based on these means, fewer patients would have been operated on the basis of the 3D measurements, in comparison to CT if the same cut-off values would have been used. Consequently, for the 3D scan EHI to be used in the process of decision-making new threshold values should be determined. To date, no such studies exist.
The study of Uccheddu et al. (17) compared the 3D scan derived ECI and the CT-scan based correction index, and found similar mean index values 17.50% (SD: 6.25%; range: 10–25%) vs. 17.00% (SD: 6.48%; range: 9–24%) with a correlation coefficient of 0.99. One may subsequently assume that both imaging modalities can be used interchangeably to determine the (external) correction index. However, the power of evidence is low with only 4 included participants, therefore more data are needed to be able to draw a definite conclusion.
The novel 3D scan-based Pectus Surface Area Ratio and Volume Ratio that were introduced by Bliss et al. (18) demonstrated only moderate correlations with the conventional HI. This may be due to the fact that the HI is calculated from a single plane that exhibits the maximum depression. Yet, pectus morphologies are not restricted to a two-dimensional plane but are rather multiplanar. The HI is, moreover, dependent of the shape of the thorax. This means that a certain pectus depth results in different indices if the chest is for example flat or barrel shaped. In the past years, several alternative metrics have been proposed to better describe the extent of PE deformities, however, their clinical use remains uncertain. Until now, the HI is still considered the reference standard for research purposes and reimbursement decisions. Nevertheless, considerations to determine surgical therapy are multifactorial and vary widely among institutions around the world. In our opinion indices should not be used as a hard criterion to determine surgical candidacy or its reimbursement but be part of the multifactorial decision wherein more attention is given to physiological symptoms such as cardiac and pulmonary impairment. To quantify cardiac function one may for example use the cardiac index, which was investigated by Maagaard et al. (4) and shown to increase following minimally invasive PE repair.
One of the main disadvantages of 3D scanning versus cross-sectional imaging is the missing intrathoracic anatomical information, such as sternal torsion and (cardio)pulmonary impression. However, as the majority of cases are not severe, they do not necessarily require cross-sectional imaging and a 3D optical image would suffice. Additional cross-sectional imaging could then be reserved for severe cases that are suspected for intrathoracic anomalies of the underlying heart and lungs. Cardiopulmonary impairment may also be assessed functionally by e.g., an electrocardiogram (ECG), echocardiography and spirometry, but their relation to cross-sectional imaging is yet to be investigated. Hypothetically, such diagnostics may even outperform conventional cross-sectional imaging as it provides dynamic information on (cardio)pulmonary functioning. For example, heart valve diseases and pulmonary function can neither be assessed by CT nor plain radiography nor with a 3D scan. It should subsequently be advised to offer functional tests such as ECG, echocardiography and spirometry or body plethysmography as adjunct methods to the standard preoperative panel, regardless of the imaging technique used to determine pectus severity. Another limitation is that 3D scans rely on body constitution. Measures in obese and female (because of the mammae) patients may subsequently differ from thin and male patients. Nevertheless, in contrast to cross-sectional images, 3D scans can be repeated endlessly without exposure to ionizing radiation. In most centers, 3D optical scanners are not available, whereas the vast majority features equipment to acquire CT-scans and plain radiographies. However, a reduction in exposure to radiation may easily justify the one-time costs of a 3D scanner.
During the respiratory cycle, chest dimensions are dynamic with the minimum anteroposterior diameter being achieved following full expiration. At this point, the external and internal HI [i.e., (E)HI] are maximum, as also found by Birkemeier et al. (20) and Albertal et al. (21) who, moreover, reported PE severity indices to be significantly more severe at end-expiration. In this review, only Uccheddu et al. (17) acquired their 3D scans at end-expiration. In addition, no studies reported the respiratory phase in which the CT was acquired. Reported index values may subsequently be an underestimation.
None of the included studies compared their 3D scan severity measurements to those based on chest radiographies. Still, standard two-view chest radiographies are commonly acquired in the daily work- and follow-up of pectus patients and serve as a valid alternative to CT-scans (22). Despite a reduction in dose compared to CT, chest radiographies still require exposure to radiation that is associated with long-term side effects ranging from growth derangements to malignancies (6,7). Following similar dogmas of minimization of radiation exposure and its potential harm, chest radiographies should ideally be replaced by a radiation free imaging method, such as 3D optical surface scanning. MRI could also serve as a radiation free alternative to radiographies and CT-scans. Its feasibility has already been demonstrated by Birkemeier et al. (23), while Lo Piccolo and colleagues (24) even found comparable severity values, comparing MRI and CT-scans. However, MRI is generally associated with increased costs, reduced availability, is more time-consuming, difficult to perform in claustrophobic patients, motion sensitive, and requires sedation in young patients, making it a less attractive alternative (23,24).
In conclusion, 3D optical scanning is an attractive, feasible and promising imaging technique to determine the severity of PE without exposure to ionizing radiation. No evidence was found that supports nor discards the use of 3D scans to determine PC severity. Meta-analytical review of participants with PE demonstrated a pooled correlation of 0.89 between the CT derived HI and its 3D scan equivalent based on external measures. However, despite this high correlation, further research is imperative for 3D scans to be used in the clinical process of decision making and help determine surgical candidacy.
Acknowledgments
The authors would like to gratefully acknowledge Marion Heijmans of the Knowledge & Information Centre (KIC), Zuyderland Medical Centre, Sittard-Geleen & Heerlen, the Netherlands for her effort and contribution to the scientific databases and registries searches.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.31). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chung CS, Myrianthopoulos NC. Factors affecting risks of congenital malformations. I. Analysis of epidemiologic factors in congenital malformations. Report from the Collaborative Perinatal Project. Birth Defects Orig Artic Ser 1975;11:1-22. [PubMed]
- Desmarais TJ, Keller MS. Pectus carinatum. Curr Opin Pediatr 2013;25:375-81. [Crossref] [PubMed]
- Kelly RE Jr, Cash TF, Shamberger RC, et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics 2008;122:1218-22. [Crossref] [PubMed]
- Maagaard M, Tang M, Ringgaard S, et al. Normalized cardiopulmonary exercise function in patients with pectus excavatum three years after operation. Ann Thorac Surg 2013;96:272-8. [Crossref] [PubMed]
- Haller JA Jr, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 1987;22:904-6. [Crossref] [PubMed]
- Brenner D, Elliston C, Hall E, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001;176:289-96. [Crossref] [PubMed]
- Don S. Radiosensitivity of children: potential for overexposure in CR and DR and magnitude of doses in ordinary radiographic examinations. Pediatr Radiol 2004;34 Suppl 3:S167-72; discussion S234-41.
- Sarwar ZU, DeFlorio R, O'Connor SC. Pectus excavatum: current imaging techniques and opportunities for dose reduction. Semin Ultrasound CT MR 2014;35:374-81. [Crossref] [PubMed]
- Pazos V, Cheriet F, Song L, et al. Accuracy assessment of human trunk surface 3D reconstructions from an optical digitising system. Med Biol Eng Comput 2005;43:11-5. [Crossref] [PubMed]
- Grant CA, Johnston M, Adam CJ, et al. Accuracy of 3D surface scanners for clinical torso and spinal deformity assessment. Med Eng Phys 2019;63:63-71. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 2012;24:69-71. [PubMed]
- National Heart L, and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Glinkowski W, Sitnik R, Witkowski M, et al. Method of pectus excavatum measurement based on structured light technique. J Biomed Opt 2009;14:044041. [Crossref] [PubMed]
- Hebal F, Port E, Hunter CJ, et al. A novel technique to measure severity of pediatric pectus excavatum using white light scanning. J Pediatr Surg 2019;54:656-62. [Crossref] [PubMed]
- Poncet P, Kravarusic D, Richart T, et al. Clinical impact of optical imaging with 3-D reconstruction of torso topography in common anterior chest wall anomalies. J Pediatr Surg 2007;42:898-903. [Crossref] [PubMed]
- Uccheddu F, Ghionzoli M, Volpe Y, et al. A novel objective approach to the external measurement of pectus excavatum severity by means of an optical device. Ann Thorac Surg 2018;106:221-7. [Crossref] [PubMed]
- Bliss DP Jr, Vaughan NA, Walk RM, et al. Non-radiographic severity measurement of pectus excavatum. J Surg Res 2019;233:376-80. [Crossref] [PubMed]
- Wong KE, Gorton GE 3rd, Tashjian DB, et al. Evaluation of the treatment of pectus carinatum with compressive orthotic bracing using three dimensional body scans. J Pediatr Surg 2014;49:924-7. [Crossref] [PubMed]
- Birkemeier KL, Podberesky DJ, Salisbury S, et al. Breathe in.. breathe out.. stop breathing: does phase of respiration affect the Haller index in patients with pectus excavatum? AJR Am J Roentgenol 2011;197:W934-9. [Crossref] [PubMed]
- Albertal M, Vallejos J, Bellia G, et al. Changes in chest compression indexes with breathing underestimate surgical candidacy in patients with pectus excavatum: a computed tomography pilot study. J Pediatr Surg 2013;48:2011-6. [Crossref] [PubMed]
- Mueller C, Saint-Vil D, Bouchard S. Chest x-ray as a primary modality for preoperative imaging of pectus excavatum. J Pediatr Surg 2008;43:71-3. [Crossref] [PubMed]
- Birkemeier KL, Podberesky DJ, Salisbury S, et al. Limited, fast magnetic resonance imaging as an alternative for preoperative evaluation of pectus excavatum: a feasibility study. J Thorac Imaging 2012;27:393-7. [Crossref] [PubMed]
- Lo Piccolo R, Bongini U, Basile M, et al. Chest fast MRI: an imaging alternative on pre-operative evaluation of Pectus Excavatum. J Pediatr Surg 2012;47:485-9. [Crossref] [PubMed]