
Transthoracic versus intra-operative transesophageal echocardiography in right heart assessment
Introduction
Tricuspid regurgitation (TR) is a common echocardiographic finding, with the prevalence of moderate or greater (≥2+) TR in this group estimated at 15% to 20% (1). Advanced stages of TR increase morbidity and mortality in left-sided valvulopathies, heart failure with preserved or reduced ejection fraction, and pulmonary hypertension, commensurate with TR severity (2,3). In patients undergoing mitral valve surgery, the presence of preoperative ≥2+ TR is associated with worse perioperative outcomes, a higher incidence of persistent TR and development of right ventricular (RV) dysfunction, and a 53% increased risk of mortality at long-term follow-up (4,5). The performance of concomitant tricuspid valve repair, in appropriate candidates with mitral valve disease undergoing surgical intervention, decreases heart failure symptoms, prevents TR progression, and improves RV dysfunction (4-6).
Given the above, the 2014 American College of Cardiology/American Heart Association and 2012 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines on the management of patients with valvular heart disease have assigned a class I indication for tricuspid valve intervention at the time of left-sided valve surgery when there is severe primary or secondary TR (7,8). Nevertheless, the absence of preoperative ≥2+ TR has poor sensitivity and negative predictive value for the occurrence of late significant TR (9). Since tricuspid annular (TA) dilatation occurs early in the disease process and is one marker of TR burden, it is recommended to consider double valve surgery not only based on the degree of TR, but also if there is significant TA remodeling and dilatation defined as an end-diastolic diameter ≥40 mm (21 mm/m2) in the two-dimensional transthoracic apical four-chamber view (2D-TTE A4C), or by direct intraoperative surgical measurement >70 mm (7,8,10,11).
Intraoperative transesophageal echocardiography (TEE) is pivotal in the assessment of valve pathology and disease severity prior to surgical intervention. Although 3D-TEE has shown excellent correlation with 2D-TTE, the modality has not been routinely adopted in the operative setting (9). Conversely, limited data exists evaluating the correlation between 2D-TEE and established 2D-TTE measures of TA, tricuspid valve, and RV geometry and function, despite its guideline-directed use (12). The current study aimed to assess the correlation between echocardiographic parameters of TA, tricuspid valve, and RV size and function as assessed by 2D-TTE and 2D-TEE.
Methods
Patient selection
This study was approved by the Institutional Review Board at Mount Sinai Medical Center (Miami Beach, FL, USA) in accordance with institutional regulations and the ethical guidelines of the 1975 declaration of Helsinki. We retrospectively analyzed the Mount Sinai Medical center echocardiography database to identify patients who underwent combined tricuspid and mitral valve surgery from 2008 to 2016. A total of 174 patients were identified, of which 54 had a preoperative 2D-TTE and intraoperative 2D-TEE available for comprehensive analysis and comprised the study cohort. The documented history and physical examination, surgical operative report, and consultation and progress notes were thoroughly reviewed to document demographic data, medical history, and peri-operative outcomes. The etiology of TR was deemed to be primary if the valve lesion was due to a leaflet abnormality, and secondary (functional) if it was the result of left-sided heart disease, TA dilatation, or RV remodeling and dilatation resulting in incomplete systolic closure. Similarly, mitral regurgitation was primary in the setting a leaflet abnormality and secondary (functional) if a sequela of left ventricular remodeling and papillary muscle displacement (7,8).
Two-dimensional transthoracic and transesophageal echocardiographic analysis
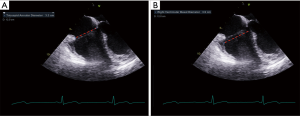
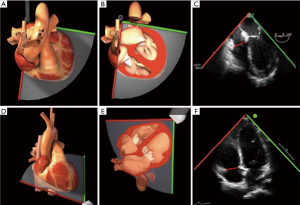
2D-TTE and TEE were performed using a General Electric ultrasound system (GE Healthcare, Chicago, IL, USA). The echocardiographic variables of interest were measured offline by two independent, blinded echocardiographers utilizing ECHOPAC software (GE Healthcare, Chicago IL, USA) in accordance with the American Society of Echocardiography guidelines on chamber quantification, and evaluation of native valvular regurgitation (10,12). The TA, tricuspid valve, and RV geometry, dimensions, and function were assessed in four commonly-acquired echocardiographic views: the 2D-TTE A4C and parasternal RV inflow views, and the 2D-TEE mid-esophageal 4-chamber (ME4C) and transgastric RV inflow views (Figure 1). Measurements were correlated between corresponding TTE and TEE views based on the orientation of septal-lateral (A4C and ME4C) and anterior-posterior anatomy (parasternal and transgastric RV inflow). The TA diameter (TAd) and RV diameter (RVd) were measured at end-diastole in all views, while the tricuspid valve tenting area (the area enclosed by the TA plane and leaflets) and the tenting height (distance from the annular plane to the leaflet coaptation point) were obtained in the apical and mid-esophageal views at mid-systole, as previously described (13). Finally, the TA plane systolic excursion (TAPSE) was assessed using M-mode echocardiography in A4C and ME4C views and aligning the cursor with the lateral TA.
Statistical methods
Continuous variables are expressed as the mean ±1 standard deviation (SD), or median and interquartile range (IQR, 25–75), as appropriate. Categorical variables are reported as frequencies and percentages. Correlations between the echocardiographic variables describing TA, tricuspid valve, and RV geometry, dimensions, and function were assessed using the Spearman’s rank correlation coefficient and results were graphically presented in scatterplots. A P value <0.05 (two-tailed) was the threshold for statistical significance. The Statistical Program for Social Sciences software (SPSS version 21.0, SPSS Inc., Chicago, IL, USA) was used to perform the analyses.
Results
Baseline and operative characteristics
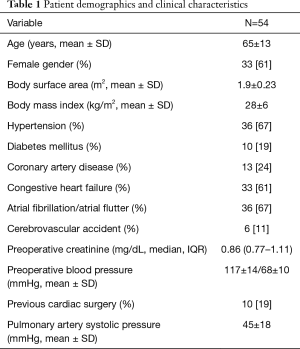
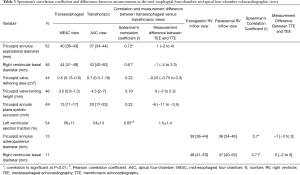
The mean age was 65±13 years and 33 (61%) were female. The most prevalent co-morbidities were a history of atrial fibrillation/flutter (67%), essential hypertension (67%), and congestive heart failure (61%). Ten (19%) patients had a history of prior valve surgery, including 2 (4%) that had undergone combined coronary artery bypass graft and valve surgery. Of these, 2 (4%) patients had recurrent mitral regurgitation status post ring annuloplasty mitral valve repair, 2 (4%) had degenerated mitral valve prostheses, and 1 (2%) had degenerated mitral and tricuspid valve prostheses (Table 1).
Full table
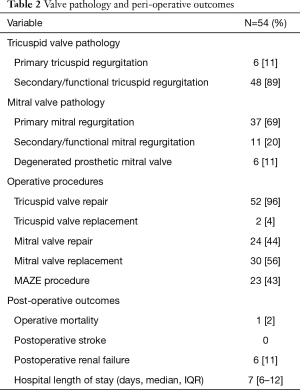
The most common pathology of tricuspid valve dysfunction was secondary/functional TR in 48 (89%) patients, while for the mitral valve was primary mitral regurgitation in 37 (69%). Valve repair was achieved in 52 (96%) and 24 (44%) of patients in the tricuspid and mitral positions, respectively. There was 1 (2%) operative mortality due to multi-organ failure and the median hospital length of stay was 7 days [interquartile range (IQR), 6–11] (Table 2).
Full table
Echocardiographic analyses
TA septolateral dilatation (diameter >35 mm) was present in 36 (67%) patients, of which 21 (39%) had significant dilatation ≥40 mm, and 34 (63%) patients had RV dilatation. The mean left ventricular ejection fraction by TTE measured 54%±13%, and was moderately correlated with the visually-estimated ejection fraction of 56%±11% by TEE (Pearson’s correlation coefficient =0.65; mean difference between TEE versus TEE =1.5%±1.4%; P<0.01).
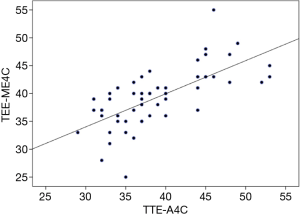
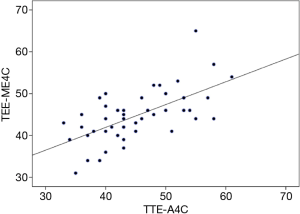
Measurement of the TA septolateral diameter in both the ME4C and A4C views was feasible in 52 (96%) patients, and for the RV basal diameter was obtainable in 46 (85%). The TA septolateral diameter strongly correlated between the two imaging modalities [Spearman’s correlation coefficient (r) =0.72], with a median overestimation of 1 mm (IQR, −2 to 4) by TEE (Figures 2,3). Measurement of the RV basal diameter in the TEE ME4C moderately correlated with TTE A4C-derived values (r=0.61), with a median underestimation of −1 mm (IQR, −4 to 3.3) utilizing TEE (P<0.01 for both) (Figure 4). No relationships between the ME4C and A4C measures of tricuspid valve geometry or TA plane systolic excursion were observed. Finally, the RV inflow-derived TA anteroposterior diameter and RV basal diameter was feasible in both the parasternal and transgastric views in 28% and 20% of patients, respectively. There were strong correlations between the TEE and TTE measurements for both the TA anteroposterior diameter (r=0.7; median underestimation by TEE =−1 mm, IQR −3 to 3) and RV basal diameter (r=0.71; median difference between TEE versus TTE =0 mm, IQR −2 to 6) (P<0.01 for both) (Table 3).
Full table
Discussion
In summary, in the present study the following important observations regarding two-dimensional TTE and TEE-derived measures of TA, tricuspid valve, and RV geometry, dimensions, and function were noted: (I) in the vast majority of patients measurements were feasible in the ME4C and A4C views, while only approximately 25% of patients had adequate imaging windows for analysis in both the parasternal and transgastric RV inflow views; (II) measurement of the TA diameter was strongly correlated between TEE and TTE for both the septolateral and anteroposterior dimensions in the ME4C-A4C and RV inflow transgastric-parasternal views, respectively; and, (III) the RV basal diameter was strongly correlated between the TTE and TEE RV inflow views, and moderately correlated when measured in the ME4C-A4C windows. Of note, these findings are placed within the context of a select group of patients with pathologic mitral and tricuspid valves, and in whom paired measurements were not available in 100% of the cohort, which limits the generalizability of the results.
A class I or IIa recommendation for surgical intervention is assigned by the 2014 American Heart Association/American College of Cardiology and the 2017 European Society of Cardiology/European Society of Cardio-Thoracic Surgery guidelines for the management of valvular heart disease to patients with severe primary or secondary TR undergoing left-sided valve surgery, and for markedly symptomatic isolated primary TR, with the preferred technique being tricuspid valve repair (7,8). In patients with less than severe secondary TR, the consideration of tricuspid valve intervention at the time of left-sided valve surgery is centered on three criteria: (I) a preoperative TA septolateral diameter >40 mm by echocardiography or >70 mm by direct intraoperative measurement; (II) progressive RV dilatation or dysfunction; or, (III) clinical signs of right heart failure (7,8). Given the poor prognosis conferred by progressive TR, and persistent or worsening valve insufficiency after left-sided surgery, the proper selection of candidates who may benefit from tricuspid valve intervention is prudent (1-3,13-15).
Despite societal guidelines, significant knowledge gaps remain regarding tricuspid valve and right heart size and function assessment utilizing TEE. Dreyfus and colleagues prospectively analyzed 282 patients referred for 2D-TTE and TEE, of which 183 had 3D-TEE imaging performed and 120 underwent combined mitral and tricuspid valve surgery. The authors found that the 2D-TTE A4C view was the most feasible and reproducible measurement of TA diameter. Despite its strong correlation with 3D-TEE there was a systematic underestimation of the TA size by 4 mm, and furthermore, correlated only modestly with direct intra-operative assessment which was poorly reproducible (9). In the present analysis, the 2D-TEE TA diameter measured at end-diastole in the ME4C view was a useful 2D-TEE marker of TA size, and accurately represented TA dilatation and remodeling with minimal overestimation. In regards to right heart size and function, the American Society of Echocardiography guidelines for performing a comprehensive TEE state that “there are inadequate data to make specific recommendations for values for RV size and function by TEE,” with RV size being judged ‘normal’ if visually estimated as less than two-thirds the size of the left ventricle (16,17). While it was found that measurement in the ME4C view by TEE underestimated the RV basal diameter by a median of 1 mm as compared with A4C 2D-TTE, the parameters correlated only modestly, and further research into 2D and 3D-TEE quantification of the right heart is warranted (18,19).
In addition to the aforementioned limited generalizability of the data presented, the small sample size and retrospective nature of the study also confer an inherent selection bias. This is furthered by data attrition due to suboptimal echocardiographic windows in a small proportion of patients when examining the measurements made in the 2D-TTE A4C and TEE ME4C views, and in a more substantial number of subjects as it pertains to the RV inflow transgastric-parasternal views. As a whole these factors limit the statistical power of intergroup and correlative analyses, and may predispose to significant type II statistical error (20). Finally, there is no echocardiographic or societal consensus regarding the TEE imaging planes which correspond to established TTE measurements of tricuspid valve and RV geometry and function.
In conclusion, 2D-TEE appears to be a reliable modality for quantitative assessment of TA and RV size and geometry when compared with guideline-directed measurements by 2D-TTE, which are frequently relied upon for intra-operative surgical decision-making at the time of mitral valve surgery or isolated tricuspid valve intervention. The current findings are best interpreted as hypothesis-generating for future external validation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christos G. Mihos) for the series “Novel Concepts in Cardiopulmonary and Structural Heart Disease” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.04). The series “Novel Concepts in Cardiopulmonary and Structural Heart Disease” was commissioned by the editorial office without any funding or sponsorship. CGM served as the unpaid Guest Editor of the series and serves as an unpaid editorial member of Journal of Thoracic Disease from Jan 2019 to Dec 2020. FN serves as an unpaid editorial member of Journal of Thoracic Disease from Aug 2019 to Jul 2021. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board at Mount Sinai Medical Center (Miami Beach, FL, USA) in accordance with institutional regulations and the ethical guidelines of the 1975 declaration of Helsinki.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405-9. [Crossref] [PubMed]
- Hung J, Koelling T, Semigran MJ, et al. Usefulness of echocardiographic determined tricuspid regurgitation in predicting event-free survival in severe heart failure secondary to idiopathic-dilated cardiomyopathy or to ischemic cardiomyopathy. Am J Cardiol 1998;82:1301-3. [Crossref] [PubMed]
- Topilsky Y, Khanna A, Toumeau T, Le , et al. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ Cardiovasc Imaging 2012;5:314-23. [Crossref] [PubMed]
- Chan V, Burwash IG, Lam BK, et al. Clinical and echocardiographic impact of functional tricuspid regurgitation repair at the time of mitral valve replacement. Ann Thorac Surg 2009;88:1209-15. [Crossref] [PubMed]
- Desai RR, Vargas Abello LM, Klein AL, et al. Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure. J Thorac Cardiovasc Surg 2013;146:1126-32.e10. [Crossref] [PubMed]
- Ueno T, Sakata R, Shigehisa Y, et al. Tricuspid regurgitation after mitral valve repair for degenerative mitral regurgitation. Ann Thorac Cardiovasc Surg 2014;20:155-60. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease : A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Dreyfus J, Durand-Viel G, Raffoul R, et al. Comparison of 2-dimensional, 3-dimensional, and surgical measurements of the tricuspid annulus size clinical implications. Circ Cardiovasc Imaging 2015;8:e003241. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Miglioranza MH, Mihaila S, Muraru D, et al. Variability of tricuspid annulus diameter measurement in healthy volunteers. JACC Cardiovasc Imaging 2015;8:864-6. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography Developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Kim HK, Kim YJ, Park JS, et al. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol 2006;98:236-42. [Crossref] [PubMed]
- Matsuyama K, Matsumoto M, Sugita T, et al. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg 2003;75:1826-8. [Crossref] [PubMed]
- Porter A, Shapira Y, Wurzel M, et al. Tricuspid regurgitation late after mitral valve replacement: clinical and echocardiographic evaluation. J Heart Valve Dis 1999;8:57-62. [PubMed]
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921-64. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. [Crossref] [PubMed]
- Amsallem M, Mercier O, Kobayashi Y, et al. Forgotten no more: A focused update on the right ventricle in cardiovascular disease. JACC Heart Fail 2018;6:891-903. [Crossref] [PubMed]
- Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012;25:3-46. [Crossref] [PubMed]
- Pocock SJ, McMurray JJ, Collier TJ. Making sense of statistics in clinical trial reports: part 1 of a 4-Part series on statistics for clinical trials. J Am Coll Cardiol 2015;66:2536-49. [Crossref] [PubMed]


