
Initial diagnosis and management of adult community-acquired pneumonia: a 5-day prospective study in Shanghai
Introduction
Community-acquired pneumonia (CAP) is a common lower respiratory infectious disease, which is a leading cause of mortality worldwide (1-3). To optimize the management of CAP in adults, guidelines are developed in different countries and regions, which are in line with the local conditions, e.g., Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) guideline (4) in America, The National Institute for Health and Care Excellence (NICE) guideline (5) in England, and the Japanese Respiratory Society (JRS) guideline (6) in Japan. According to the pathogen spectra of CAP and available diagnostic tools and therapeutics in China, the Chinese Thoracic Society (CTS) updated the clinical practice guideline for CAP in adults in 2016, including many aspects such as diagnostic criteria, severity evaluation, treatment, and follow-up assessment (7).
Some research showed that compliance with guidelines can improve clinical outcomes in CAP patients (8,9). However, adherence to regional CAP guidelines was unsatisfactory in many countries (10). Overdiagnosis of pneumonia (11), lack of CURB-65 severity score documentation (C: disturbance of consciousness, U: urea nitrogen, R: respiratory rate, B: blood pressure, 65: age) and microbiological tests (12), unreasonable prescription of initial empirical antibiotics (13), as well as neglect of vaccination (14) are often observed in many clinical audits of CAP. However, data on the actual practices in adult CAP management in China are scarce.
Therefore, through this study, we aimed to reflect the current profile of adult CAP management through a quick, 5-day audit. With 2/3 of outpatients and 1/3 of inpatients recruited, we also aimed to identify the problems in real clinical settings and provide evidence for future improvement.
Methods
Study setting
This was a short-term, prospective, cross-sectional observational study, encompassing all the administrative districts of Shanghai, China. We used the cluster sample method to select 46 pulmonologists from 36 hospitals, located in different administrative districts of Shanghai. These 46 pulmonologists were asked to record the management and prognosis data of all the adult CAP patients who they cared for during the consecutive five work days, from January 8, 2018 to January 12, 2018. The participating centers are listed in the Acknowledgement section.
Participants
All adult patients who were cared for by the 46 pulmonologists during the study period and met the following inclusion criteria were recruited: (I) onset in community; (II) chest imaging indicating emerging pulmonary interstitial or parenchyma changes, with or without pleural effusion; (III) at least one of the following clinical manifestations: (i) new onset of cough or expectoration, or exacerbation of existing respiratory symptoms, with or without hemoptysis, dyspnea, purulent sputum, or chest pain; (ii) fever; (iii) signs of pulmonary consolidation and/or moist rales; (iv) increase or decline in peripheral blood leucocyte count (>10×109/L or <4×109/L).
Exclusion criteria were as follows: (I) age <18 years; (II) evidence that strongly indicated the presence of other pulmonary diseases at the first visit, including tuberculosis, pulmonary tumor, noninfectious interstitial lung disease, pulmonary edema, atelectasis, pulmonary embolism, pulmonary eosinophilia and pulmonary vasculitis.
Ethical approval
The study was approved by the Institutional Review Board of Shanghai Ruijin Hospital (Reference Number: 2017-C-186) and was conducted in accordance with the tenets of the Declaration of Helsinki, 1964, and its later amendments and Good Clinical Practice guidelines. Informed consent was obtained from all the enrolled patients.
Measurements
Data pertaining to the following variables were collected: (I) the diagnostic information (demographic data, clinical manifestations, underlying diseases, and chest imaging); (II) severity evaluation (CURB-65, CRB-65 score, and oxygenation assessment); (III) treatment (site-of-care decisions, antibiotic regimens, drug administration route, and duration of therapy); (IV) prognostic assessment (time to clinical stabilization and 30-day mortality).
We did not collect data on microbiological tests because it was generally dispensable for the outpatients with CAP at a low risk of death (15). Data were collected via the online electronic questionnaire star platform (https://www.wjx.cn/, Changsha Haoxing Information Technology Co., Ltd., China). The follow-up visit on the 30th day since the diagnosis of CAP was accomplished via telephone conversation.
Definitions
The definition and calculation method of CRB-65 and CURB-65 are described in the previous guideline (5).
We recorded the following comorbidities: tumor, diabetes, congestive heart failure, cerebrovascular diseases, hepatic diseases, renal diseases, asthma, chronic obstructive pulmonary disease, interstitial pulmonary disease, bronchiectasis, recent injury or surgery history, nasogastric intubation, and immunocompromised status.
Immunocompromised status was defined as meeting one or more of the following criteria: (I) taking glucocorticoids (prednisone 0.3 mg/kg/d or equivalent doses of other kinds of steroids) for more than 3 weeks; (II) neutrophil deficiency (neutrophil counts <500/µL) for more than 10 days; (III) having undergone allogeneic hematopoietic stem cell transplantation; (IV) taking immunosuppressive agents or nucleoside analogues within 90 days; (V) having acquired immune deficiency syndrome; (VI) having combined or congenital immunodeficiency syndrome, such as chronic granulomatosis.
Oxygenation status was determined by any of the recorded results of the non-invasive methods (pulse oximetry monitoring or signs of cyanosis) or the invasive method (arterial blood gas analysis). Hypoxemia (16) was defined as meeting one of the following criteria: (I) recorded cyanosis, (II) pulse O2 saturation <90%, (III) partial pressure of oxygen in arterial blood <60 mmHg or oxygenation index <300 when breathing air.
Clinical stabilization was defined as meeting all of the following criteria (7): (I) body temperature ≤37.8 °C; (II) heart rate ≤100 bpm; (III) respiratory rate ≤24 bpm; (IV) systemic blood pressure ≥90 mmHg; (V) O2 saturation ≥90% on room air.
Initial treatment failure was defined as the fulfilment of either of the following criteria (7): (I) unrelieved symptoms after initial therapy requiring alternative antibiotics; (II) disease progression and deterioration during initial therapy or after initial improvement.
Statistical analysis
Owing to the nature of a clinical audit, predesigned sample size calculation was not warranted in the present study. All the continuous variables were expressed as mean ± standard deviation. Normality was analyzed using the Kolmogorov-Smirnov test. The Student’s t-test or Mann-Whitney U tests were used on the basis of data distribution to compare continuous variables between two subgroups. All categorical data were described as constituent ratios and analyzed using Pearson’s Chi-Squared test. All statistical analyses were performed using SPSS 23.0 (SPSS Inc, Chicago, IL, USA). A two-tailed P<0.05 was considered to be statistically significant.
Results
In this short period, 5-day survey, a total of 459 participants were enrolled in accordance with the inclusion criteria. Twenty-four patients were withdrawn due to loss to follow-up by the 30th day. Therefore, 435 patients were included in the final analysis, with 41.4% from tertiary hospitals (n=180), 57.0% from secondary hospitals (n=248), and 1.6% from primary hospitals (n=7). Overall, 17.2% of the participants were diagnosed with CAP in the Emergency Room (n=75) and 78.6% in the Outpatient Department (n=342).
Baseline characteristics of patients
The proportion of females (54.7%) was slightly higher than that of males (45.3%). The average age was 58.4 (range, 18–97) years. The 18–40-year age group accounted for 18.9%, the 41–65-year age group for 47.8%, and the elderly (>65 years) for 33.3% of the participants. Among patients with CRB-65 records (n=415, 95.4%), 59.1% (n=257) had a CRB-65 score of 0, 35.2% (n=153) a score of 1, and 1.1% (n=5) a score of 2, indicating that the majority of the patients were at a low risk of death (CRB-65 score: 0–1).
Concomitant diseases of the participants included diabetes (10.1%), cerebrovascular diseases (4.1%, implying aspiration risks), hepatic diseases (3.2%), asthma (3.2%), chronic obstructive pulmonary disease (2.75%), and tumor (2.53%). (only comorbidities with >2% proportion are shown). Only 3 patients were immunocompromised. Of the above comorbidities, only the hepatic disease was associated with treatment failure (P=0.0487, odds ratio =3.17, 95% confidence index =1.04–9.92).
CRB-65 plus oxygenation assessment may be more suitable than CURB-65 for the initial evaluation of severity
We observed that the CURB-65 calculation was not performed for 305 (70.1%) patients at the time of diagnosis, although it was widely recommended to predict mortality and to help to decide site-of-care by guidelines (4,7). In comparison, only 4.6% (n=20) of the patients lacked the calculation of CRB-65 score. The practicability of CRB-65 was better than that of CURB-65 across different clinical settings or departments (Figure 1).
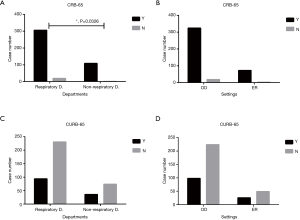
Oxygenation status was assessed for most of the patients (92.9%), and 19 of them suffered from hypoxemia. The significant difference in the time to clinical stability demonstrated that patients with hypoxemia were more severe and more difficult to treat than those with normal oxygenation (8.42±6.36 vs. 5.53±4.12 days, P=0.004). In addition, Table 1 showed that both CRB-65 and CURB-65 were not sensitive enough to detect hypoxemia in patients who were at a low risk of death.

Full table
Overtreatment was relatively common in patients at a low risk of death
After being diagnosed with CAP, 68.3% of patients received ambulatory treatments, and 31.7% of the patients were admitted to hospital. As shown in Table 2, the proportion of hospitalization increased correspondingly to the increase in CRB-65 score. Nevertheless, 27.6% of patients (n=71) with a CRB-65 score of 0 were still hospitalized, which violated the general recommendation in guidelines. The median age of these patients was 56 (interquartile range, 41–61) years. In addition, only 8.2% (n=6) of these hospitalized patients had hypoxemia and 26.0% (n=19) had at least one comorbidity (Table 3). Therefore, we speculated that nearly 1/5 (n=50) of the patients with a CRB-65 score of 0 may be inappropriately admitted to hospital.

Full table

Full table
In addition, a majority of patients (n=373, 85.7%) in our population were administered antibiotics intravenously, and 84.4% of patients with a CRB-65 score of 0 received intravenous antibiotics (Table 2). However, the oral and intravenous antibiotic administration groups had similar initial treatment failure rates (15.2% vs. 16.9%, P=0.730) in the whole study population, implying that the efficacy of oral administration should not have been ignored from the perspectives of both clinical outcomes and guideline recommendations. Moreover, the total antibiotic duration was 10.4±4.89 days and intravenous antibiotic duration was 8.89±3.95 days. There was no significant difference in the total antibiotic duration between different severity grades (P=0.067, Table 2). A slight increase in the intravenous duration was observed between CRB-65 score 0 and score 1 grade (P=0.011, Table 2). We thought that the total antibiotic duration of patients at a low risk of death was relatively unreasonable and should be shorter than that of patients with a high CRB-65 score.
Empirical antibiotic therapy encompassing atypical pathogens showed faster response but not shortened duration
The overview of initial empirical antibiotic regimen is shown in Table 4. A relatively high proportion of the patients (n=186, 42.8%) received combination antibiotic therapy, and 72.6% received antibiotics covering atypical pathogens. In addition, 13 patients received antiviral therapy, and 5 patients had viral pneumonia, which was confirmed by laboratory tests. After 3 days of initial therapy, the antibacterial regimen of 67 (15.4%) patients was changed because of treatment failure.

Full table
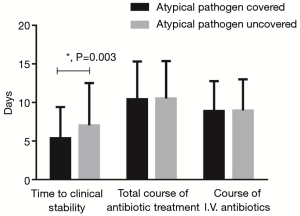
As Figure 2 shows, the time to clinical stability of patients receiving antibiotics that encompassed atypical pathogens (n=316, 5.36±4.05 days) was significantly decreased compared with those not receiving the coverage (n=118, 7.03±5.49 days, P=0.003). No significant difference in the rate of treatment failure was observed between two groups, with a decreasing trend in the atypical coverage group (13.9% vs. 19.5%, P=0.329). However, there was no difference in the total duration of antibiotic treatment between the two groups (10.42±4.91 vs. 10.53±4.84 days, P=0.853). The course of antibiotic intravenous administration was also similar (8.87±3.91 vs. 8.95±4.06 days, P=0.429). Furthermore, no significant difference in age, blood leukocyte count, blood neutrophil percentage, C-reactive protein, symptoms, comorbidities, and chest image manifestations was observed between the two groups, except for sex. Hence, the physicians seemingly had no such understanding that patients who received antibiotics encompassing atypical pathogens and achieved clinical stability early should timely discontinue the antibiotics.
Discussion
Through this quick 5-day prospective study in Shanghai, we have two major findings. First, CRB-65 combined with oxygenation assessment seemed to be more practical than CURB-65 for the initial evaluation of CAP. Second, we found that some of the patients at a low risk of death might be over-treated, especially in terms of hospitalization and antibiotic prescription.
CURB-65 is a widely accepted severity assessment tool for CAP, effectively predicting 30-day mortality (17). However, it does not seem to be well-adopted by clinicians in our study, probably due to the fact that blood urea nitrogen level was not routinely tested in patients who presented with cough and sputum with or without fever. Similar to our study, the low appliance of CURB-65 also observed in another clinical audit of a European Gaza hospital (12). Considering that CRB-65 was more frequently used in our study, we thought CRB-65 was more practical and acceptable than CURB-65 for initial evaluation in China, and the NICE guideline had the same recommendation (5). In addition, CURB-65 was less effective in predicting the prognosis of influenza pneumonia (18), but the inefficiency could be offset by the advantage of oxygenation assessment in predicting the mortality of influenza pneumonia (19). Apart from influenza pneumonia, we found that the oxygenation status was associated with the time to clinical stability in our population. Another study also suggested that timely oxygenation assessment was helpful in reducing the mortality of severe CAP (20), and Japan has already included oxygen saturation into its national assessment tool of CAP, A-DROP score (21). Therefore, considering the feasibility of CRB-65 and the efficacy of oxygenation assessment (22), we could replace CURB-65 with CRB-65 plus oxygenation assessment for initial CAP evaluation in the primary care settings of China.
As for potential overtreatment, about 1/5 of patients with a CRB-65 score of 0 were still hospitalized after excluding hypoxemia and other comorbidities (23). Another retrospective study in China (24) also demonstrated that a large number of patients at a low risk of death were admitted to hospital. Excessive hospitalization not only results in heavy financial burden to both patients and the national medical insurance system, but also increases the risks of hospital-acquired infections (25,26). Apart from CAP, approximately 244 million patients were admitted to hospital in 2017 nationally (26), implying the phenomenon of excessive hospitalization. The payment policy of the national medical insurance might account for the improper hospitalization rate for these patients at a low risk of death. In addition, it was prevalent for patients at a low risk of death to receive intravenous antibiotics. Similar initial treatment failure rates in the oral and intravenous administration groups were observed in our study, indicating that intravenous antibiotic administration for patients at a low risk of death could not bring about increased clinical outcomes. Therefore, patients at a low risk of death who can be treated in outpatient settings according to the CRB-65 or CURB-65 score should be encouraged to receive oral antibiotic therapy as far as possible, as many guidelines recommended (7).
Furthermore, we observed that empirical antibiotic therapy encompassing atypical pathogens seemed highly efficient in shortening time to clinical stability, but the antibiotic duration was not correspondingly shortened. An epidemiological survey in China demonstrated that the atypical pathogen infection accounted for nearly half of CAP cases (27), which could explain its advantage. However, the total and intravenous duration of atypical coverage therapy were similar to those without coverage, which was unreasonable and should be adjusted. Some randomized controlled trials also proved the noninferiority of a short-course antimicrobial regimen in terms of clinical stability, bacterial eradication, and mortality, compared with an extended-course regimen (28,29). Furthermore, many guidelines (3-5,7) advocated an early switch from intravenous to oral administration and then timely discontinuation of antibiotic therapy when clinical stability was achieved. Therefore, Chinese physicians should be educated further to prescribe shorter intravenous duration of antibiotics as well as total duration for patients receiving atypical coverage.
The strengths of our study were that we prospectively collected data of CAP patients, including those who were treated in ambulatory settings. Most cross-sectional surveys of CAP were targeted for inpatients (13,14,24,30), but nearly 80% of CAP patients are treated in the ambulatory setting (31). In our study, 94.3% of patients were at a low risk of death according to CRB-65 score and more than half of them were treated in the outpatient setting, which bridged the gap in the existing literature on CAP patients with a low risk of death, in China. Perhaps our conclusion could be generalized to the initial diagnosis and treatment of CAP in other regions with discretion. What’s more, this short-term prospective survey effectively identified some problems in CAP management, including low usage rate of CURB-65, possible excessive hospitalization, and delayed switch from intravenous to oral antibiotics.
Our study had some limitations. First, the cross-sectional design made it difficult to prove the causality of hypotheses, and the number of hypoxemia events was relatively small. Thus, we plan to enroll more patients with hypoxemia and poor outcomes in the subsequent cohort to prove the efficacy of CRB-65 plus oxygenation assessment in predicting poor outcomes and evaluating severity using the receiver operating characteristic curve method. Moreover, we could not explain some confounders. For instance, hospital admission decision can also be influenced by the physician’s determination of some factors besides pneumonia severity, including comorbidities of patients and the availability of family and primary care support (4,32). However, we excluded the influence of comorbidity and hypoxemia, and found that about 1/5 of the patients with a CRB-65 score of 0 were inappropriately hospitalized. Intravenous administration of antibiotics can also be attributed to noncompliance with oral therapy and abnormal gastrointestinal tract functioning apart from simple medical overuse (4). We will complement these factors in the subsequent survey. The short duration of 5 days might lead to the season bias. Nevertheless, the Centre for Disease Control in Shanghai did not report any small epidemics of unusual pathogens in January, 2018.
Conclusions
We conclude that CRB-65 may be more practical than CURB-65 for the initial assessment of CAP. Oxygenation assessment should be included in the evaluation. Overtreatment in patients at a low risk of death is relatively common, requiring targeted improvement. More efforts should be made to improve compliance with guidelines, with a focus on encouraging oral antibiotic therapy in the ambulatory setting for patients at a low risk of death, shortening antibiotic duration, and driving a timely switch from intravenous to oral therapy.
Acknowledgments
All authors would like to acknowledge the following physicians and hospitals participating in this cross-sectional clinical audit, of which the name is listed in the alphabetical order of the family name: Hui-Fang Cao, Jingan District Central Hospital, Shanghai; Yuan-Jing Chen, Kongjiang Hospital, Shanghai; De-Jie Chu, The Eighth People’s Hospital, Shanghai; Qi-Jian Cheng, Ruijin North Hospital, Shanghai; Ke-Wen Cheng, Renhe Hospital, Shanghai; Chun-Lin Du, Qingpu Branch of Zhongshan Hospital Affiliated To Fudan University, Shanghai; Jing-Qing Hang, Putuo District People’s Hospital, Shanghai; Qin Jia, Shanghai East Hospital, Shanghai; Zhi-Jun Jie, The Fifth People’s Hospital, Shanghai; Xiao-Yan Jin, Tongren Hospital, Shanghai; Fan Li, Songjiang District Central Hospital, Shanghai; Xing-Jing Li, Baoshan Branch of The First People’s Hospital, Shanghai; Wen-Jie Li, Beizhan Hospital, Shanghai; Ping Liu, The First Rehabilitation Hospital, Shanghai; Li-Wen Lu, Fengxian District Central Hospital, Shanghai; Yong Luo, Chongming Branch of Xinhua Hospital, Shanghai; Jia-Yun Ma, The Third People’s Hospital, Shanghai; Wei-Feng Pi, Jiangong Hospital, Shanghai; Yu-Lin Qian, Luodian Hospital, Shanghai; Ye-Chang Qian, Baoshan District Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai; Chun-Lin Tu, Jiading District Central Hospital, Shanghai; Xiong-Biao Wang, Putuo District Central Hospital, Shanghai; Jin Wang, Shanghai North Hospital, Shanghai; Xiao-Ru Wang, Dahua Hospital, Shanghai; Ling Wang, Jiangwan Hospital, Shanghai; Qian Wang, Zhabei District Central Hospital, Shanghai; Guo-Zhong Yao, The General Hospital of Shanghai Corps, Shanghai; Rong-Huan Yu, Xuhui District Central Hospital, Shanghai; Yun-Feng Zhao, Punan Hospital, Shanghai; Lei Zhao, Pudong New Area Gongli Hospital, Shanghai; Jing Zhang, Zhongshan Hospital, Shanghai; Yi-Hui Zhang, The First People’s Hospital Branch, Shanghai; Bo-Ying Zhang, Luwan Branch of Ruijin Hospital, Shanghai; Cui-Xia Zheng, Yangpu District Central Hospital, Shanghai; Qi-Xing Zhou, Liqun Hospital, Shanghai; An-Shan Zhuo, People Pla 411 Hospital, Shanghai.
Funding: The study is supported by the program of Shanghai key discipline for respiratory diseases (Number 2017ZZ02014) and the National Innovative Research Team of High-level Local Universities in Shanghai.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Shanghai Ruijin Hospital (Reference Number: 2017-C-186) and was conducted in accordance with the tenets of the Declaration of Helsinki, 1964, and its later amendments and Good Clinical Practice guidelines. Informed consent was obtained from all the enrolled patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Almirall J, Bolíbar I, Vidal J, et al. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J 2000;15:757-63. [Crossref] [PubMed]
- Raut M, Schein J, Mody S, et al. Estimating the economic impact of a half-day reduction in length of hospital stay among patients with community-acquired pneumonia in the US. Curr Med Res Opin 2009;25:2151-7. [Crossref] [PubMed]
- Takaki M, Nakama T, Ishida M, et al. High incidence of community-acquired pneumonia among rapidly aging population in Japan: a prospective hospital-based surveillance. Jpn J Infect Dis 2014;67:269-75. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27-72. [Crossref] [PubMed]
- Eccles S, Pincus C, Higgins B, et al. Diagnosis and management of community and hospital acquired pneumonia in adults: summary of NICE guidance. BMJ 2014;349:g6722. [Crossref] [PubMed]
- Miyashita N, Matsushima T, Oka M, et al. The JRS guidelines for the management of community-acquired pneumonia in adults: an update and new recommendations. Intern Med 2006;45:419-28. [Crossref] [PubMed]
- Cao B, Huang Y, She DY, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J 2018;12:1320-60. [Crossref] [PubMed]
- Mortensen EM, Restrepo M, Anzueto A, et al. Effects of guideline-concordant antimicrobial therapy on mortality among patients with community-acquired pneumonia. Am J Med 2004;117:726-31. [Crossref] [PubMed]
- Capelastegui A, España PP, Quintana JM, et al. Improvement of process-of-care and outcomes after implementing a guideline for the management of community-acquired pneumonia: a controlled before-and-after design study. Clin Infect Dis 2004;39:955-63. [Crossref] [PubMed]
- Priya D, Thomas B, Sally W, et al. Adult community acquired pneumonia (CAP) audit report national audit period: 1 December 2014–31 January 2015. British Thoracic Society, 2015. Available online: https://www.brit-thoracic.org.uk/document-library/quality-improvement/audit-reports/adult-community-acquired-pneumonia-201415/. Accessed July 6, 2019.
- Daniel P, Bewick T, Welham S, et al. Adults miscoded and misdiagnosed as having pneumonia: results from the British Thoracic Society pneumonia audit. Thorax 2017;72:376-9. [Crossref] [PubMed]
- Alyacoubi S, Abuowda Y, Albarqouni L, et al. Inpatient management of community-acquired pneumonia at the European Gaza Hospital: a clinical audit. Lancet 2018;391 Suppl 2:S40. [Crossref] [PubMed]
- Lim WS, Woodhead M. British Thoracic Society. British Thoracic Society adult community acquired pneumonia audit 2009/10. Thorax 2011;66:548-9. [Crossref] [PubMed]
- Al-Abri SS, Al-Maashani S, Memish ZA, et al. An audit of inpatient management of community-acquired pneumonia in Oman: a comparison with regional clinical guidelines. J Infect Public Health 2012;5:250-6. [Crossref] [PubMed]
- Theerthakarai R, El-Halees W, Ismail M, et al. Nonvalue of the initial microbiological studies in the management of nonsevere community-acquired pneumonia. Chest 2001;119:181-4. [Crossref] [PubMed]
- España PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med 2006;174:1249-56. [Crossref] [PubMed]
- Chalmers JD, Singanayagam A, Akram AR, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 2010;65:878-83. [Crossref] [PubMed]
- Mulrennan S, Tempone SS, Ling IT, et al. Pandemic influenza (H1N1) 2009 pneumonia: CURB-65 score for predicting severity and nasopharyngeal sampling for diagnosis are unreliable. PLoS One 2010;5:e12849. [Crossref] [PubMed]
- Shi SJ, Li H, Liu M, et al. Mortality prediction to hospitalized patients with influenza pneumonia: PO2/FiO2 combined lymphocyte count is the answer. Clin Respir J 2017;11:352-60. [Crossref] [PubMed]
- Blot SI, Rodriguez A, Solé-Violán J, et al. Effects of delayed oxygenation assessment on time to antibiotic delivery and mortality in patients with severe community-acquired pneumonia. Crit Care Med 2007;35:2509-14. [Crossref] [PubMed]
- Shindo Y, Sato S, Maruyama E, et al. Comparison of severity scoring systems A-DROP and CURB-65 for community-acquired pneumonia. Respirology 2008;13:731-5. [Crossref] [PubMed]
- Kolditz M, Ewig S, Schütte H, et al. Assessment of oxygenation and comorbidities improves outcome prediction in patients with community-acquired pneumonia with a low CRB-65 score. J Intern Med 2015;278:193-202. [Crossref] [PubMed]
- Cillóniz C, Polverino E, Ewig S, et al. Impact of age and comorbidity on cause and outcome in community-acquired pneumonia. Chest 2013;144:999-1007. [Crossref] [PubMed]
- Chen L, Zhou F, Li H, et al. Disease characteristics and management of hospitalised adolescents and adults with community-acquired pneumonia in China: a retrospective multicentre survey. BMJ Open 2018;8:e018709. [Crossref] [PubMed]
- Zingg W, Holmes A, Dettenkofer M, et al. Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. Lancet Infect Dis 2015;15:212-24. [Crossref] [PubMed]
- National Health Commission of The People’s Republic of China. The 2017 Statistical Bulletin on Development of Health Care in China. Available online: http://www.nhfpc.gov.cn/guihuaxxs/s10743/201806/44e3cdfe11fa4c7f928c879d435b6a18.shtml
- Tao LL, Hu BJ, He LX, et al. Etiology and antimicrobial resistance of community-acquired pneumonia in adult patients in China. Chin Med J (Engl) 2012;125:2967-72. [PubMed]
- Pinzone MR, Cacopardo B, Abbo L, et al. Duration of antimicrobial therapy in community acquired pneumonia: less is more. ScientificWorldJournal 2014;2014:759138. [Crossref] [PubMed]
- Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med 2016;176:1257-65. [Crossref] [PubMed]
- Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415-27. [Crossref] [PubMed]
- Mandell LA, Marrie TJ, Grossman RF, et al. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Canadian Community-Acquired Pneumonia Working Group. Clin Infect Dis 2000;31:383-421. [Crossref] [PubMed]
- Marras TK, Gutierrez C, Chan CK. Applying a prediction rule to identify low-risk patients with community-acquired pneumonia. Chest 2000;118:1339-43. [Crossref] [PubMed]


