
Standard and extended sleeve resections of the tracheobronchial tree
Introduction
Anatomic resections with bronchial and/or vascular resections and reconstruction, so called sleeve resections were originally performed in patients with impaired cardio-pulmonary reserves. This technique enabled the avoidance of pneumonectomy. Later on, it was realized that pneumonectomy represents a disease itself (1). Nowadays, lung-sparing procedures are the treatment of choice also in patients without impaired cardiopulmonary reserves and advanced nodal disease whenever technically possible (2). In this review article we set out to provide a contemporary overview on this topic.
Definitions and types of sleeve resection
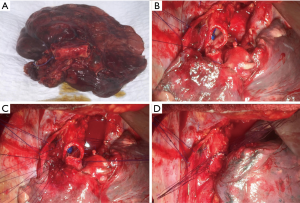
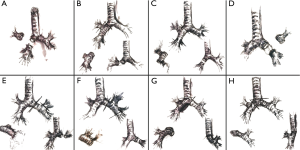
Standard sleeve resection is defined as a surgery to remove one lobe and a part of the main airway/bronchus. Afterwards, anastomosis is performed between the both ends of the bronchi (Figures 1,2A). Sleeve resection can be defined as autotransplantation for salvage of the remaining healthy lung parenchyma. The same procedure can be also done at the level of the pulmonary artery (PA), defined as broncho-vascular sleeve resection or double sleeve resection. Common sleeve resections are shown in Figure 2A,B,C,D,E,F,G,H.
On this basis, extended sleeve resection is defined as a bronchoplastic procedure with resection more than a lobe in terms of additional wedge resections or segmentectomies at the remaining lobe(s) (3,4). According to Okada and Barthet, extended sleeve resections can be classified into four groups (5,6).
Type A
Resection of the upper and middle lobes (with or without segment 6) and anastomosis between the right main and the lower lobe bronchus (or basal segmental bronchus) (Figure 2C).
Type B
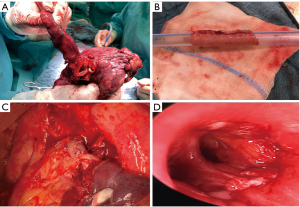
Resection of the upper lobe and superior segment of the lower lobe and anastomosis between the left main and basal segmental bronchi (Figures 2F,3).
Type C
Resection of the lingular segment and lower lobe as well as anastomosis between the left main and upper division bronchi.
Type D
Resection of the middle and the lower lobe and anastomosis between the right main and superior bronchi (Figure 2B).
The authors propose another extended sleeve resection.
Type E
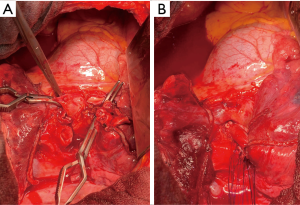
En-bloc resection of the middle lobe and segment 6 as well as anastomosis between the Bronchus intermedius and the basal segmental bronchus (Figure 4).
Vogt-Moykopf et al. described further possibilities for the salvage of lung parenchyma (7):
- Lobar transposition in terms of double sleeve upper bilobectomy with additional anastomosis of the inferior pulmonary vein to the place of the superior pulmonary vein.
- Pneumonectomy followed by ex situ sleeve resection and autotransplantation of lobe(s) or segment(s).
In some cases, isolated bronchial sleeve resections without the loss of lung parenchyma are indicated and feasible. The most common indication is a carcinoid tumor, but other malignant or benign tumors as well as trauma were also conceivable (8). The most common resections are as follows:
- Resection of the right main bronchus and end-to-end anastomosis between the distal trachea and the distal right main bronchus.
- Resection of the intermediate bronchus and end-to-end anastomosis.
- Resection of the left main bronchus and end-to-end anastomosis between the distal trachea and the distal left main bronchus.
However, there are many other possibilities for totally parenchyma-sparing bronchial sleeve resection (8).
Patients with centrally located limited disease lung cancer or other entities involving the carina can still be candidates for surgery. In these cases, the dissection of the distal trachea including the carina and reconstruction of the trachea and the main bronchus is necessary for a complete resection, so called sleeve pneumonectomy (Figures 2G,H,5). To avoid anastomotic tension, the limit of resection between the distal trachea and the main bronchus is considered to be 4 cm (9).
Indications
Lung cancer or metastatic hilar lymph node involvement of the origin of the lobar bronchus and/or the PA are the most common indications for sleeve resections. It can also be necessary to perform a sleeve resection in case of pulmonary metastases, benign tumors as well as traumatic ruptures or posttraumatic or inflammatory strictures (10,11).
Preoperative assessment
All patients should undergo preoperative cardiopulmonary evaluation (12). Inhalative preoperative treatment might enhance pulmonary function and reduce postoperative pulmonary complications in patients with newly diagnosed COPD during the preoperative assessment (13). After pulmonary rehabilitation, more patients might be considered fit for surgery and thus being able to receive optimal oncological therapy for lung cancer. An oncological staging is also important for patients undergoing oncological resection (11).
A bronchoscopy should be performed by the surgeon to evaluate the precise location of the tumor. In the setting of multimodality treatment including surgery, the bronchoscopy should be performed before and after neoadjuvant therapy, since a complete resection is important even if the tumor is no longer visible after neoadjuvant therapy. Special attention should be paid to patients with chronic use of steroids, patients with excessive dynamic airway collapse as well as neoadjuvant radiochemotherapy, since it might lead to impairment of anastomotic healing (14-16).
During the operation, maintaining adequate gas exchange is often quite demanding and close and effective cooperation between the anesthesiologist and the surgeon is necessary. Cross-field ventilation of the remaining bronchus as well as high-frequency jet ventilation can be used for oxygenation during the resection and reconstruction period (17). Extracorporeal membrane oxygenation (ECMO) has gained popularity in oncological airway surgery in recent years (18). However, the anesthetic considerations are reviewed elsewhere (19).
Techniques of tracheo-bronchial resection and anastomosis
No irreversible steps should be taken before resectability is confirmed. Thus, full intraoperative evaluation of intra- and extraluminal growth of the tumor, full mobilization and dissection of the involved bronchi and the branches of the PA, as well as intraoperative bronchoscopy are an essential condition sine qua non. For any type of sleeve resection, complete lymphadenectomy and hilar dissection should be performed before the phases of resection and reconstruction in order to avoid any traction and damage on the anastomosis in the late period of the surgical procedure. A semicircular pericardiotomy and the mobilization of the hilum allows easy creation of tension-free anastomosis. Nonetheless, skeletalizing the bronchus and possible impairment of the bronchial blood supply should be avoided. Importantly, possible thermal damage to the vascularization should be avoided by not using electrocautery near the bronchus.
The authors prefer a knife for the inter-cartilagilinar bronchial resection to obtain straight and well vascularized margins. However, other experts prefer an incision of the bronchus with a scalpel and completion of the bronchial resection with a scissor, especially in videothoracoscopic and robotic-assisted sleeve resections, respectively. Any rough edges or projecting cartilage should be trimmed. Intra-operative frozen section examination should be carried out to ensure tumor free margins.
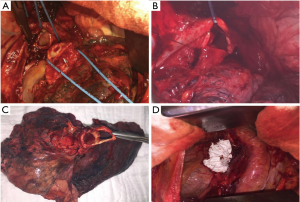
The anastomosis is performed edge-to-edge and end-to-end, respectively. The authors prefer interrupted 4-0 sutures (PDS, Ethicon Inc., Norderstedt, Germany) for the complete bronchial anastomoses. Other suture size and material might be used depending on the anastomoses to be performed and surgeons’ preference. The sutures are placed around the cartilages at each side of the anastomosis, passing through the full thickness of the bronchial wall. At the beginning, two sutures are placed at the dorsal wall of the anastomosis and knotted (Figure 1B). Further sutures are placed from posteriorly to anteriorly and the knots are placed extra luminary. The sutures are left untied until all sutures are placed correctly (Figure 1C). At the end, the sutures are tied beginning from anterior to posterior where the first knots were done (Figure 1D) (11). Continuous suture is another possibility for the bronchial anastomosis, especially in minimally-invasive approach (20). On the other hand, some experts prefer continuous suture for the pars membranacea and interrupted sutures for the pars cartilaginea.
If there is great discrepancy between the sizes of the bronchi, various surgical techniques might help to resolve the problem of high-caliber mismatch, especially in extended sleeve resections (5,21,22):
- Telescope anastomosis (smaller distal bronchus is inserted into the larger proximal bronchus).
- Bronchial stump adaption (larger proximal bronchus is narrowed at the level of the pars membranacea).
- Oblique resection of the distal bronchus (in order to increase the circumference of the smaller distal bronchus).
Techniques of protection of anastomosis
Bronchial anastomotic dehiscence has an incidence of 5–8.7% after sleeve lobectomy (6). Dehiscence represents one of the major and often lethal complications after bronchial sleeve resection due to possible erosion of the adjacent PA and massive hemorrhage. Therefore, some authors recommend the protection of the bronchial anastomosis site. Various tissue has been used for the protection (23):
- Intercostal pedicle flap;
- Pericardial pedicle flap;
- Pleural flap;
- Pericardial fat pad graft;
- Omentum;
- Thymic flap;
- Pedicled pericardiophrenic graft.
For all cases, the blood circulation of the used tissue has to be maintained and the thickness of the tissue has to be adequate not to compromise the adjacent PA. Contrary, there are advocates of unprotected bronchial anastomosis, even after induction treatment (2,24).
Techniques of resection and reconstruction of the PA
Resection and reconstruction of the PA might be indicated whenever the tumor itself or lymph node metastases do not allow sufficient access to ligate the lobar and/or segmental arteries separately. Not rarely, bronchovascular sleeve resection of the upper lobe including a sleeve resection of the PA may become necessary because of the close anatomical relationship between the left PA and the left upper lobe bronchus (Figure 1D). The sleeve resection of the PA may also be required to prevent a kinking of the artery caused by the longitudinal length difference between the shortened bronchus and the artery (10,25). Isolated resection and reconstruction of the PA might be reliable technique to apply parenchyma-saving lung surgery.
Full control over the PA (intra- or extra-pericardially) should be obtained whenever resection and reconstruction of the PA seem to be necessary. The PA should be mobilized to its origin to accommodate an atraumatic clamp. The authors’ preference is administration of Heparin i.v. (5,000 IU) prior to clamping the main PA. Other experts prefer lower dosage of heparin (1,500–2,000 IU) (26). The second clamp is placed distally to the planed resection of the PA to control back bleeding. We prefer the clamping of the pulmonary vein to have enough place for the planed procedure.
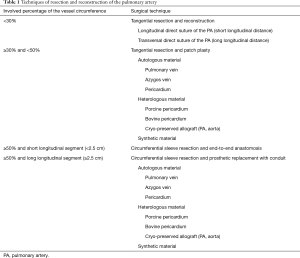
An overview of different techniques of resection and reconstruction of the PA are presented in Table 1. The simplest way for angioplasty is the tangential resection of the PA with direct suture (27). Occasionally, transversal suturing might be necessary to avoid stenosis and/or kinking of the PA. However, some authors consider direct suturing of the PA not as vascular reconstruction (28).
Full table
If the involved percentage of the vessel circumference is 330% and <50%, tangential resection and patch plasty represents the surgical techniques of choice. Circumferential sleeve resection and end-to-end anastomosis is carried out whenever the involved percentage of the vessel circumference is 350% and the resected longitudinal segment is short (<2.5 cm). Any size mismatch of the vessels can be accommodated by oblique division of the smaller vessel or by incising along the lumen of the distal vessel. In case of longer distances, there are different possibilities to achieve tension-free vascular anastomosis: release of the pulmonary ligament, mobilization of the hilum, pericardiotomy and intrapericardial dissection, respectively (11). The authors prefer the division of the Botalli ligament to bridge the necessary distance. Whenever the distance can’t be bridge with release maneuvers, prosthetic replacement of the PA with conduit might solve the problem (Figure 3). Different autologous and heterogenous as well as synthetic materials can be used for plasty and conduit as shown in Table 1 (29-34).
Arterial reconstructions and anastomoses are usually performed using running sutures (5-0 Prolene, Ethicon Inc., Norderstedt, Germany). In Case of conduit reconstruction, proximal anastomosis should be completed first. Second anastomosis should be finished after size adjustment of the conduit. Of note, arterial anastomosis is performed upon completion of the bronchial anastomosis in event of required bronchovascular sleeve resection. The special role of postoperative anticoagulation after reconstruction of the PA is unclear. On one hand, there is a risk of bleeding. On the other hand, there is the risk of clotting and lung infarction at the PA reconstruction site. Therefore, some authors recommend low-dose heparinization during the hospitalization. Anyway, postoperative anticoagulation prophylaxis is required for the prevention of venous thromboembolism after lung cancer surgery for 4 weeks according to guidelines, irrespective of reconstruction of the PA (35).
Postoperative management and complications
Due to interruption of parasympathic nerves and lymphatic vessels, sleeve resection is associated with hypersecretion of mucus and impaired mucociliary clearance (36). Before extubation, a bronchoscopy should be performed for endobronchial clearance as well as anastomosis inspection. However, the patients should be extubated following the operation. Depending on the extend of the resection, residual pleural space management might be important.
During the postoperative course, bronchoscopy should be performed in case of retention of secretions and to monitor the healing of the anastomosis (37). Anastomotic complications like necrosis, micro-fistula or dehiscence can be detected during bronchoscopy. After sleeve lobectomy, anastomotic dehiscence usually leads to a secondary pneumonectomy. Completion pneumonectomy is a high-risk procedure and is characterized by high mortality rates (11). Bölükbas et al. reported the first case of a secondary sleeve resection of the lingula and anastomosis between the left main bronchus and the apical trisegment group (B1-3) following bronchial anastomotic dehiscence after left lower lobe sleeve resection to avoid a secondary pneumonectomy (38). However, prophylaxis, further postoperative complications, classifications and management of the complications are reviewed by Ludwig et al. (39).
Outcomes of sleeve resections
In a meta-analysis of almost 3,000 patients, sleeve lobectomy was associated with better outcomes compared to pneumonectomy in terms of operative mortality (3% vs. 6%), locoregional recurrence (17% vs. 30%) and five-year survival (50% vs. 30%), respectively (40). Quality of life and functional outcomes play an important role for the patients faced to various treatment options in case of lung cancer. Analyses of quality of life are in favor of sleeve resections compared to pneumonectomy (41,42). Furthermore, sleeve lobectomy was favored with regard to quality-adjusted life years in a meta-analysis with 99 investigated studies comparing pneumonectomy to sleeve resections (43). In this context, many studies have proven that sleeve lobectomy is associated with less loss of pulmonary function after sleeve lobectomy (44,45).
Conclusions
In conclusion, sleeve resection is an established surgical procedure of first choice for tracheobronchial pathologies, whenever anatomically and oncologically feasible. Experienced thoracic surgeons have a broad surgical armentarium to avoid the “iatrogenic disease” of pneumonectomy. Sleeve resections are associated with better outcomes in all aspects. Thus, sleeve resection is not an alternative for pneumonectomy and vice versa.
Acknowledgments
We thank Dr. Uwe Bauer for drawing the schematic representation of selected sleeve resections of the bronchus.
Funding: None.
Footnote
Provenance and Peer review: This article was commissioned by the Guest Editor (Servet Bölükbas) for the series “Airway Surgery”, published in Journal of Thoracic Disease. This article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.65). The series “Airway Surgery” was commissioned by the editorial office without any funding or sponsorship. SB served as the unpaid Guest Editor for the series and serves as the unpaid editorial board of Journal of Thoracic Disease from Nov 2018 to Oct 2020. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bölükbas S, Eberlein M, Schirren J. Pneumonectomy vs. Sleeve Resection for Non-Small Cell Lung Carcinoma in the Elderly: Analysis of Short-term and Long-term Results. Thorac Cardiovasc Surg 2011;59:142-7. [Crossref] [PubMed]
- Schirren J, Kudelin N, Fischer A, et al. The Role of Sleeve Resections in Advanced Nodal Disease. Eur J Cardiothorac Surg 2011;40:1157-63. [Crossref] [PubMed]
- Johnston JB, Jones PH. The treatment of bronchial carcinoma by lobectomy and sleeve resection of the main bronchus. Thorax 1959;14:48-54. [Crossref] [PubMed]
- Sagawa M, Aikawa H, Usuda K, et al. Extended sleeve pulmonary resection in a patient with synchronous triple bronchogenic squamous cell carcinoma. Lung Cancer 2008;59:262-5. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Extended sleeve lobectomy for lung cancer: the avoidance of pneumonectomy. J Thorac Cardiovasc Surg 1999;118:710-3. [Crossref] [PubMed]
- Berthet JP, Paradela M, Jimenez MJ, et al. Extended sleeve lobectomy: one more step toward avoiding pneumonectomy in centrally located lung cancer. Ann Thorac Surg 2013;96:1988-97. [Crossref] [PubMed]
- Vogt-Moykopf I, Trainer S, Schirren J. Sleeve lobectomy. In: Shields TW (ed): General Thoracic Surgery Vol 1. 4th edition. Philadelphia: Williams & Wilkins, 1994:452-60.
- Bölükbas S, Schirren J. Parenchyma-Sparing Bronchial Sleeve Resections in Trauma, Benign and Malign Diseases. Thorac Cardiovasc Surg 2010;58:32-7. [Crossref] [PubMed]
- De Perrot M. Tracheal Sleeve Pneumonectomy. In: Shields TH, LoCicero J, Reed CE, et al. editors. General Thoracic Surgery. 7th Edition. Ambler: Lippincott Williams & Wilkins, 2009:471-8.
- Vogt-Moykopf I, Toomes H, Heinrich S. Sleeve Resection of the Bronchus and Pulmonary Artery for Pulmonary Lesions. Thorac Cardiovasc Surg 1983;31:193-8. [Crossref] [PubMed]
- Bölükbas S, Ghezel-Ahmedi D, Kudelin N, et al. Sleeve resections for the treatment of non-small cell lung cancer. Minerva Chir 2011;66:329-39. [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy. Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Bölükbas S, Eberlein M, Eckhoff J, et al. Short-term effects of inhalative tiotropium/formoterol vs. tiotropium/formoterol/budenoside in surgery patients with newly diagnosed COPD: a prospective randomized trial. Eur J Cardiothorac Surg 2011;39:995-1000. [Crossref] [PubMed]
- Bölükbas S, Bergmann T, Fisseler-Eckhoff A, et al. Short- and Long-term Outcome of Sleeve Resections in the Elderly. Eur J Cardiothorac Surg 2010;37:30-5. [Crossref] [PubMed]
- Tapias LF, Ott HC, Mathisen DJ. Complications Following Carinal Resections and Sleeve Resections. Thorac Surg Clin 2015;25:435-47. [Crossref] [PubMed]
- Koryllos A, Lopez-Pastorini A, Zalepugas D, et al. Bronchus anastomosis healing depending on type of neoadjuvant therapy. Ann Thorac Surg 2020;109:879-86. [Crossref] [PubMed]
- Weder W, Inci I. Carinal resection and sleeve pneumonectomy. J Thorac Dis 2016;8:S882-8. [Crossref] [PubMed]
- Akil A, Bölükbas S, Wiebe K. Extracorporeal Membrane Oxygenation in Thoracic Surgery: Establishing Functional and Technical Operability. Zentralbl Chir 2019;144:78-85. [PubMed]
- Schleicher A, Groeben H. Anesthetic considerations for tracheobronchial surgery. J Thorac Dis 2020;6138-42.
- Kutlu CA, Goldstraw P. Tracheobronchial sleeve resection with the use of a continuous anastomosis: results of one hundred consecutive cases. J Thorac Cardiovasc Surg 1999;117:1112. [Crossref] [PubMed]
- Hollaus PH, Janakiev D, Pridun NS. Telescope anastomosis in bronchial sleeve resections with high-caliber mismatch. Ann Thorac Surg 2001;72:357-61. [Crossref] [PubMed]
- Hong TH, Cho JH, Shin S, et al. Extended sleeve lobectomy for centrally located non-small-cell lung cancer: a 20-year single-centre experience. Eur J Cardiothorac Surg 2018;54:142-8. [Crossref] [PubMed]
- Venuta F, Diso D, Anile M, et al. Techniques of protection and revascularization of the bronchial anastomosis. J Thorac Dis 2016;8:S181-5. [PubMed]
- Storelli E, Tutic M, Kestenholz P, et al. Sleeve resections with unprotected bronchial anastomoses are safe even after neoadjuvant therapy. Eur J Cardiothorac Surg 2012;42:77-81. [Crossref] [PubMed]
- Nakajima D, Oda H, Chen-Yoshikawa TF, et al. Emergent surgical treatment for acute thrombosis caused by pulmonary artery kinking after left upper sleeve lobectomy. Interact Cardiovasc Thorac Surg 2019;29:481-3. [Crossref] [PubMed]
- D’Andrilli A, Maurizi G, Ciccone AM, et al. Long-segment pulmonary artery resection to avoid pneumonectomy: long-term results after prosthetic replacement. Eur J Cardiothorac Surg 2018;53:331-5. [Crossref] [PubMed]
- Vannucci J, Matricardi A, Potenza R, et al. Lobectomy with angioplasty: which is the best technique for pulmonary artery reconstruction? J Thorac Dis 2018;10:S1892-8. [Crossref] [PubMed]
- Shrager JB, Lambright ES, McGrath CM, et al. Lobectomy with tangential pulmonary artery resection without regard to pulmonary function. Ann Thorac Surg 2000;70:234-9. [Crossref] [PubMed]
- Rendina EA, Venuta F. Reconstruction of the pulmonary artery. In: Patterson GA (ed). Thoracic Surgery, 3rd edn. Philadelphia: Churchill Livingstone, 2009:909-22.
- Rendina EA, Venuta F, De Giacomo T, et al. Reconstruction of the pulmonary artery by a conduit of autologous pericardium. J Thorac Cardiovasc Surg 1995;110:867-8. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann Thorac Surg 1999;68:995-1001. [Crossref] [PubMed]
- D’Andrilli A, Venuta F, Maurizi G, et al. Bronchial and arterial sleeve resection for lung cancer after induction therapy. Thorac Surg Clin 2014;24:411-21. [Crossref] [PubMed]
- D’Andrilli A, Ibrahim M, Venuta F, et al. Glutaraldehyde preserved autologous pericardium for patch reconstruction of the pulmonary artery and superior vena cava. Ann Thorac Surg 2005;80:357-8. [Crossref] [PubMed]
- Berthet JP, Boada M, Paradela M, et al. Pulmonary sleeve resection in locally advanced cancer using cryo- preserved allograft for pulmonary artery replacement. J Thorac Cardiovasc Surg 2013;146:1191-7. [Crossref] [PubMed]
- Li H, Jiang G, Bölükbas S, Chen C, et al. The Society for Translational Medicine: the assessment and prevention of venous thromboembolism after lung cancer surgery. J Thorac Dis 2018;10:3039-53. [Crossref] [PubMed]
- Wood PB, Gilday D, Ilves R, et al. A comparison of gas exchange after simple lobectomy and lobectomy with sleeve resection in dogs. J Thorac Cardiovasc Surg 1974;68:646-53. [Crossref] [PubMed]
- Ludwig C, Stoelben E. A new classification of bronchial anastomosis after sleeve lobectomy. J Thorac Cardiovasc Surg 2012;144:808-12. [Crossref] [PubMed]
- Bölükbas S, Eberlein M, Zanner R, et al. Secondary lingular sleeve resection to avoid pneumonectomy following bronchial anastomotic dehiscence after left lower lobe sleeve resection for destroyed lung syndrome. Surg J (N Y) 2018;4:e14-7. [Crossref] [PubMed]
- Ludwig C. Prophylaxis and management of postoperative complications after tracheobronchial surgery. J Thorac Dis 2020; [Crossref]
- Stallard J, Loberg A, Dunning J, et al. Is a sleeve lobectomy significantly better than a pneumonectomy? Interact Cardiovasc Thorac Surg 2010;11:660-6. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. J Thorac Oncol 2008;3:604-8. [Crossref] [PubMed]
- Andersson SE, Rauma VH, Sihvo EI, et al. Bronchial sleeve resection or pneumonectomy for non-small cell lung cancer: a propensity-matched analysis of long-term results, survival and quality of life. J Thorac Dis 2015;7:1742-8. [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- Schirren J, Bölükbas S, Bergmann T, et al. Prospective Study on Perioperative Risks and Functional Results in bronchial and bronchovascular sleeve resections. Thorac Cardiovasc Surg 2009;57:35-41. [Crossref] [PubMed]
- Bölükbas S, Eberlein M, Schirren J. Pneumonectomy vs. Sleeve Resection for Non-Small Cell Lung Carcinoma in the Elderly: Analysis of Short-term and Long-term Results. Thorac Cardiovasc Surg 2011;59:142-7. [Crossref] [PubMed]


