
Tricuspid regurgitation and in-hospital outcomes after transcatheter aortic valve replacement in high-risk patients
Introduction
Tricuspid regurgitation (TR) is present in 15% to 20% of patients referred for echocardiography and increases mortality commensurate to the degree of valve insufficiency (1). Moreover, non-treated significant TR at the time of left-sided valve surgery is associated with post-operative morbidity and current guidelines recommend concomitant tricuspid valve intervention for patients with severe TR and/or annular dilatation (2-6).
Transcatheter aortic valve replacement (TAVR) is approved for treatment of symptomatic severe aortic stenosis (AS) in patients at low to high surgical risk. In this population the reported prevalence of moderate to severe TR ranges between 10% and 31% (7-14) and may lessen the clinical benefits of TAVR. In the PARTNER II trial (inoperable cohort), moderate or severe TR were associated with a 1.6- and 3.2-fold increased risk of post-TAVR mortality, as was right atrial or ventricular enlargement (8). Yet, the prognostic impact of TR following TAVR is uncertain and there are no clear guideline recommendations for the management of patients with severe AS and significant TR in the TAVR era.
In this study, we aimed to investigate the impact of TR on short-term TAVR outcomes and ascertain the role of clinical factors associated with TR severity after TAVR.
Methods
Data collection
This study was approved by the Institutional Review Board of Mount Sinai Medical Center. From August 2014 to January 2017, data was collected on all patients with severe AS that underwent TAVR at Mount Sinai Medical Center, Miami Beach, Florida. All patients underwent evaluation by a multidisciplinary Structural Heart Team including interventional and noninvasive cardiologists and cardiothoracic surgeons. TAVR was performed using the self-expandable Medtronic CoreValve System (Medtronic Inc., Minneapolis, MN, USA) or the balloon expandable Edwards-SAPIEN System (Edwards Lifesciences Inc., Irvine, CA, USA) at the discretion of the implanting team. Valve sizing was determined using preoperative three-dimensional transesophageal echocardiography or computed tomography. All procedures were performed in a hybrid cardiac catheterization suite under general anesthesia, under fluoroscopy and transesophageal or transthoracic echocardiographic guidance.
Prior (within 45 days) to the TAVR procedure patients had a complete transthoracic echocardiogram which was again repeated after the procedure. Measurements of left ventricular (LV) chamber dimensions, ejection fraction, LV mass, and left atrial volume were made as recommended by the American Society of Echocardiography (ASE) (15). TR severity was categorized as trace/mild (0–1+), moderate (2+), or severe (3–4+) according to ASE guidelines (16,17). Change in the severity of TR was also analyzed in three groups as follows: (I) patients who remained mild (≤1+) had pre and post TAVR TR assessed as mild; (II) patients who improved had moderate or severe TR that improved by 1 or more grades; and, (III) patients who had no change/worsened had baseline and post-TAVR persistent moderate or severe TR. TR was divided into functional (annular dilation, leaflet tethering), pacemaker mediated, or primary TR. Continuous Doppler was used to calculate the peak and mean aortic valve gradients using the modified Bernoulli equation. The aortic valve area (AVA) was calculated by the continuity equation. Stroke volume was calculated using the left ventricular outflow tract velocity integral and the left ventricular outflow tract diameter. Right ventricular systolic pressure (RVSP) was evaluated using the TR jet peak velocity and the inferior vena cava collapsibility index.
Outcomes
The main outcomes were all-cause and cardiovascular mortality as well as hospital length of stay (LOS). All-cause mortality was defined as death from any cause during hospitalization. Cardiovascular (CV) mortality was defined as mortality as a result of cardiac related causes. Hospital LOS was calculated as the difference (in days) between the date of procedure to the date of discharge or death.
Statistical methods
All continuous variables were graphed and visually assessed for normality. Normally distributed variables were expressed as mean and standard deviations of the mean (SD) while the non-normally distributed variables were expressed as median and interquartile range (IQR). Categorical variables were expressed in percentages. Median values across ordinal categories were compared with trend analysis (unless otherwise stated) while the chi-square was used to compare proportions or percentages. The student t-test was used to assess for differences in mean of normally distributed continuous variables between two categories or before and after TAVR. To compare all-cause and cardiovascular disease related mortality among TR groups, Poisson regression coefficients were computed and were transformed into risk ratios and 95% confidence interval (CI). For LOS analysis, median regression coefficients were computed. Multivariate models were adjusted for age, sex, cigarette smoking, left ventricular ejection fraction, NYHA class in the preceding two weeks, mitral regurgitation severity, a history of prior myocardial infarction and prior coronary artery bypass grafting (CABG), and The Society of Thoracic Surgeons (STS) score (Data Version 2.9; http://riskcalc.sts.org/stswebriskcalc/calculate). All analyses were conducted in Stata version 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
Results
Population characteristics
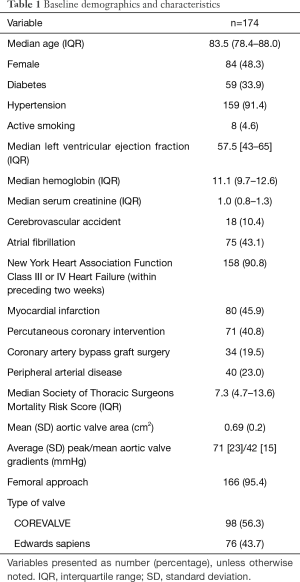
A total of 174 participants with complete pre and post TAVR data on TR were included in the study. The median age was 84 years with 48% being female. Most participants (91%) were NYHA class III or IV in the preceding 2 weeks. The median (IR) STS score was 7.3% (4.7–13.6%). Median (IR) LVEF was 57% (43–65%). The pre-procedural mean (SD) AVA was 0.69 (0.2) cm2 and the peak/mean gradients were 71 [23]/42 [15] mmHg. 98 patients (56%) received a CoreValve and the remaining of patients (44%) the Edwards-SAPIEN valve. Trans-apical approach was used in 5 patients, trans-iliac access in 2 and trans-aortic approach in one patient. For the rest of cases (95%), percutaneous-femoral access was used. Post implantation AVA and mean (SD) gradients were 1.64 (0.9) cm2 and 10 [12] mmHg, respectively. The rest of the baseline (pre-TAVR) characteristics are presented in Table 1.
Full table
TR severity before and after TAVR
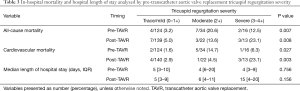
Pre TAVR, 28.7% of patients had moderate or severe TR. The majority of cases had functional TR and only three patients had mixed functional/pacemaker related etiology. After TAVR (Table 2) there was an increase in the prevalence of mild TR from 71% to 80% while moderate and severe TR decreased with TAVR from 20% to 13% and 9% to 7%, respectively (P<0.001).

Full table
TR severity and cardiovascular disease and all-cause mortalities
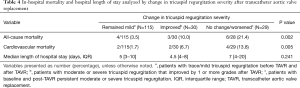
Table 3 shows the frequency of both CV and all-cause in-hospital mortalities among participants based on their pre and post-TAVR TR severity. Patients with no change/worsened (moderate or severe TR both pre and post-TAVR) had a higher frequency of all-cause and cardiovascular related-mortality when compared with those whose TR improved or remained mild (Table 4).
Full table
Full table
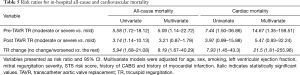
In both univariate and multivariate analysis, TR pre-TAVR was significantly associated with increased risk of CV and all-cause mortality [relative risk (RR): 14.67 (1.35–159.51) and 5.09 (1.14–22.72), respectively]. Similarly, those who experienced no change/worsened TR had a significantly increased risk of both CV and all-cause mortality when compared with improved or remained mild, even after adjusting for possible confounders. Finally, post-TAVR TR was only associated with all-cause and cardiac mortality in univariate analysis (Table 5).
Full table
TR severity and hospital LOS
As shown in Tables 3 and 4, severe post-TAVR TR showed higher hospital LOS relative to those with mild or moderate TR, however, this did not achieve statistical significance on trend analysis. In median regression analysis, there was no association between pre-TAVR TR, or change in TR after TAVR, with hospital LOS. However, those who remained with severe TR post-TAVR had a statistically significantly higher median LOS after controlling for confounders [9.9 (2.9–17.0) days]. Details can be found in Table 6.

Full table
TR severity and RVSP
Ninety-four of the 174 participants had complete data on RVSP before and after TAVR. The mean (SD) RVSP declined from 46.0 (15.3) to 40.4 (12.6) mmHg with TAVR (P<0.001) (Table 7). Among those with mild TR at baseline (n=59), the mean (SD) RVSP did not statistically change from pre to post TAVR (P=0.326). However, among those with moderate TR there was a statistically significant decline in RVSP from a mean (SD) of 55.7 (14.5) to 44.6 (13.2) mmHg (P<0.001), and from 61.6 (16.1) to 47.2 (17.2) mmHg in patients with severe TR (n=9) (P=0.072). Details can be found in Table 7. As shown in Table 8, among the remained mild and no change/worsened TR groups, there was no difference in the mean RVSP with TAVR. However, in patients with improved TR after TAVR, there was a significant reduction in the mean RVSP from 58.5 (15.6) to 40.4 (14.5) mmHg (P<0.001).

Full table

Full table
Discussion
In this cohort of high surgical risk individuals with severe AS undergoing TAVR, the following important observations were noted post-intervention: (I) approximately one-third of patients had moderate or greater TR (≥2+), of which less than half had improvement in TR with TAVR; (II) pre and post-TAVR TR severity were associated with increased in-hospital CV and all-cause mortality, and those with moderate or greater TR who remained same/worsened had the least favorable outcomes; (III) patients with severe TR post-TAVR experienced the longest hospital LOS; (IV) RVSP was independently associated with TR severity, and a reduction of RVSP after TAVR was associated with improvement in TR severity; and, (V) RVSP remained unchanged post-TAVR in those in whom TR remained same/worsened.
The prevalence of significant TR in this cohort was similar to that reported in prior studies of TAVR patients (8-14). Moreover, our findings agree with results from other observational studies reporting increased in-hospital, 30-day and one-year mortality for those patients with significant TR undergoing TAVR (8,13). In agreement with the literature (18,19), the etiology of TR in the great majority of these patients with severe AS was functional. Hence, TR was related to elevated ventricular pressures and pulmonary hypertension (PHT). TR severity can be a marker of high risk individuals. In fact, the severity of TR increases along with the risk estimated by the STS score (13). However, the presence of moderate or severe TR increased the risk of in-hospital mortality independently of the STS scores, MR severity and LV ejection fraction. Thus, moderate or severe TR is an independent risk factor for post TAVR in-hospital complications in high surgical risk individuals.
In our study, TAVR improved TR severity; however, the early salutary effect of TAVR on TR was only seen in a third of patients, and mostly in those with moderate TR (35% improvement), with a very modest effect (18% improvement) in patients with severe TR. Hence, the great majority of individuals with severe TR had persistent moderate to severe TR post-TAVR. The reported improvement of TR grade in prior studies ranges from 15% to 50% following TAVR (8-14) and surgical AVR (6). Thus, in a sizable percentage of individuals with moderate or greater TR at the time of TAVR, TR severity remains stable or worsens after the procedure (remained same/worsened). Importantly, mortality was the highest in this subgroup of patients. Interestingly, Worku et al. showed that in those with moderate or severe TR post TAVR, TR severity is unchanged or worsen after one year, and is associated with worse long-term survival (12). Hence, these observations suggest that this subgroup of individuals may benefit from additional tricuspid valve intervention.
In the present study, RVSP was higher in patients with significant TR. RVSP improved after TAVR but remained high in those in whom remained same/worsened, indicating that persistent PHT may play an important etiological role in the pathophysiology of persistent TR post-TAVR. Likewise, Worku et al. (12) and McCarthy et al. (13) found that in TAVR patients, RVSP increased along with TR severity and was associated with increased mortality (12,13). Alushi et al. investigated the role of PHT in patients undergoing TAVR (20). The authors found that in most patients post TAVR there is an early and late reduction of pulmonary pressures. Moreover, those patients with reversible PHT were at lower risk of all-cause mortality, however, in patients with residual PHT there was a higher risk of all-cause mortality at 30 days, 1 year and long-term. Importantly, absence of significant TR was an independent predictor of pulmonary pressure reduction post TAVR (20). These observations suggest that preoperative (or at the time of TAVR) right heart catheterization in a selected group of patients (e.g., those with RV dysfunction) with significant TR/elevated RVSP may help to further risk stratify and tailor medical treatment pre or immediately post-TAVR.
Limitations
This was a retrospective study and it is subject to information bias. Moreover, the analysis was conducted in a cohort of high risk individuals and it is unclear if these findings would apply to moderate or low risk TAVR patients. We relied upon incomplete hemodynamic data obtained by echocardiography to estimate RVSP, which results in a form of attrition bias. Moreover, RVSP may be underestimated in patients with severe TR. However, the association of RVSP and TR severity after TAVR remained when individuals with severe TR were excluded from the analysis. TR and RVSP were evaluated only immediately post TAVR and their severity may vary in the short-term post TAVR. However, this would not change the hospital outcomes measured in the present analysis.
Conclusions
In this cohort of high surgical risk individuals with severe AS undergoing TAVR, moderate or greater TR was associated with increased in-hospital CV and all-cause mortality. Furthermore, patients who remained with significant TR after TAVR had the least favorable outcomes. RVSP correlated with TR severity and improved after TAVR, however, RVSP remained elevated in those patients in whom TR remained significant post TAVR, suggesting that residual significant PHT contributes to significant TR and poor outcomes post TAVR.
Acknowledgments
We are very thankful to Dr. Gervasio A. Lamas for his administrative support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christos G. Mihos) for the series “Novel Concepts in Cardiopulmonary and Structural Heart Disease” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.10). The series “Novel Concepts in Cardiopulmonary and Structural Heart Disease” was commissioned by the editorial office without any funding or sponsorship. CGM served as the unpaid Guest Editor of the series and serves as an unpaid editorial member of Journal of Thoracic Disease from Jan 2019 to Dec 2020. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Mount Sinai Medical Center.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405-9. [Crossref] [PubMed]
- Di Mauro M, Bivona A, Iacò AL, et al. Mitral valve surgery for functional mitral regurgitation: prognostic role of tricuspid regurgitation. Eur J Cardiothorac Surg 2009;35:635-9. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardiothoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [Crossref] [PubMed]
- Taramasso M, Maisano F, De Bonis M, et al. Prognostic impact and late Evolution of untreated moderate (2/4+) functional tricuspid regurgitation in patients undergoing aortic valve replacement. J Card Surg 2016;31:9-14. [Crossref] [PubMed]
- Jeong DS, Sung K, Kim WS, et al. Fate of functional tricuspid regurgitation in aortic stenosis after aortic valve replacement. J Thorac Cardiovasc Surg 2014;148:1328-1333.e1. [Crossref] [PubMed]
- Rozenbaum Z, Granot Y, Steinvil A, et al. Aortic stenosis with severe tricuspid regurgitation: comparative study between conservative transcatheter aortic valve replacement and surgical aortic valve replacement combined with tricuspid repair. J Am Soc Echocardiogr 2018;31:1101-8. [Crossref] [PubMed]
- Lindman BR, Maniar HS, Jaber WA, et al. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: insights from the Placement of Aortic Transcatheter Valves II inoperable cohort. Circ Cardiovasc Interv 2015. [Crossref] [PubMed]
- Hutter A, Bleiziffer S, Richter V, et al. Transcatheter aortic valve implantation in patients with concomitant mitral and tricuspid regurgitation. Ann Thorac Surg 2013;95:77-84. [Crossref] [PubMed]
- Schwartz LA, Rozenbaum Z, Ghantous E, et al. Impact of right ventricular dysfunction and tricuspid regurgitation on outcomes in patients undergoing transcatheter aortic valve replacement. J Am Soc Echocardiogr 2017;30:36-46. [Crossref] [PubMed]
- Barbanti M, Binder RK, Dvir D, et al. Prevalence and impact of preoperative moderate/severe tricuspid regurgitation on patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2015;85:677-84. [Crossref] [PubMed]
- Worku B, Valovska MT, Elmously A, et al. Predictors of persistent tricuspid regurgitation after transcatheter aortic valve replacement in patients with baseline tricuspid regurgitation. Innovations (Phila) 2018;13:190-9. [Crossref] [PubMed]
- McCarthy FH, Vemulapalli S, Li Z, et al. Association of tricuspid regurgitation with transcatheter aortic Valve replacement outcomes: A report from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Ann Thorac Surg 2018;105:1121-8. [Crossref] [PubMed]
- Sathananthan J, Murdoch DJ, Lindman BR, et al. Implications of concomitant tricuspid regurgitation in patients undergoing transcatheter aortic valve replacement for degenerated surgical aortic bioprosthesis: Insights From the PARTNER 2 Aortic Valve-in-Valve Registry. JACC Cardiovasc Interv 2018;11:1154-60. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Zoghbi WA, Asch FM, Bruce C, et al. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2019;32:431-75. [Crossref] [PubMed]
- Hahn RT. State-of-the-art review of echocardiographic imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ Cardiovasc Imaging 2016;9:e005332. [Crossref] [PubMed]
- Mangieri A, Montalto C, Pagnesi M, et al. Mechanism and Implications of the Tricuspid Regurgitation: From the Pathophysiology to the Current and Future Therapeutic Options. Circ Cardiovasc Interv 2017;10:e005043. [Crossref] [PubMed]
- Alushi B, Beckhoff F, Leistner D, et al. Pulmonary hypertension in patients With severe aortic stenosis: Prognostic impact after transcatheter aortic valve replacement: Pulmonary hypertension in patients undergoing TAVR. JACC Cardiovasc Imaging 2019;12:591-601. [Crossref] [PubMed]


