Efficacy and safety of endobronchial valves for advanced emphysema: a meta analysis
Introduction
Emphysema is a chronic obstructive respiratory disease characterized by destruction of pulmonary elastic tissue and hyperinflated lung parenchyma. A great proportion of patients with emphysema are suffering uncontrolled dyspnoea, decreased pulmonary function and exercise tolerance, even after administration of high doses of inhaled corticosteroids (ICSs) and long acting bronchodilators. Medical therapy is often inadequate, none of the existing pharmacotherapy alone or in combination has been shown to significantly improve the long-term lung function or reduce symptom (1-3). Lung volume reduction surgery (LVRS) has been found to alleviate symptoms and improve survival rate in a subgroup of patients with heterogeneous emphysema (4-6) but the risks of morbidity and mortality are excessive. There is no effective therapy for advanced emphysema at present. Hence, to find novel therapeutic methods is needed. In the past decade, the new technology of bronchoscopic lung volume reduction with endobronchial valves (EBV) has been applied to clinical. Concerns of the less invasive bronchoscopic techniques to treat emphysema to achieve the similar beneficial effects to LVRS have been developed.
To date, a variety of noninvasive endoscopic lung volume reduction subjects became the center of clinical research and developed with the hope of improving the respiratory function of these patients. These new techniques include EBV, foam sealant, metallic coils, airway bypass stents and vapor thermal ablation (7,8), and EBV is the most common bronchoscopic lung volume reduction method to treat emphysema.
Bronchoscopic lung volume reduction with EBV are one-way blocking devices that stop entry of air into the most affected emphysematous zone during inspiration while allowing it to escape during expiration in order to induce lobar atelectasis (9).
Nowadays, there are published randomized controlled trials (RCTs) suggesting the role of EBV in emphysema patients (10-12). Some non-controlled studies also reported (13-15) clinical improvements in lung volumes, health status and exercise tolerance. However, the time of the clinical application of the new technology is not long enough, and it isn’t much mature. EBV is still being at the explorating stage, it might be to bring some complications and side effects, such as pneumothorax, hemoptysis, COPD exacerbation, death, after the valve implantation. So, the aim of this meta-analysis is to evaluate the efficacy and safety of the available latest evidence and compare EBV with standard medications and sham EBV for the treatment of emphysema patients.
Methods
Search strategy
We searched PubMed, EMBASE, CNKI, Cochrane Library database, from inception to June 2014 using the search terms “EBV” or “intrabronchial valves” or “endoscopic lung volume reduction”, “bronchoscopic lung volume reduction” and “emphysema”. We did not specify population restrictions and geographic areas, but the language only limited in English and Chinese. Data quoted as unpublished or derived from abstracts were not used. Reference lists of all primary studies and review articles were screened for additional references.
Inclusion and exclusion criteria
Studies fulfilling the following selection criteria were included in this meta-analysis: (I) emphysema patients treated with EBV and sham EBV or standard medications according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines; (II) RCTs that enrolled emphysema patients with forced expiratory volume in one second (FEV1) of 15% to 45% of the predicted value, a total lung capacity of more than 100% of the predicted value; (III) clinical efficacy or safety chosen randomly from the same geographic region; (IV) both experiments and controls should be available for estimating relative risks (RR), weighted mean differences (WMD) and 95% confidence interval (CI).
Studies were excluded if one of the following existed: (I) not relevant to EBV treatment with emphysema; (II) not randomized or controlled trials; (III) reviews and abstracts.
Study selection
Two reviewers independently scanned all titles and abstracts that indicated the study was a randomized or controlled trial evaluating the treatment with EBV among patients for advanced emphysema. The reviewers independently assessed the full text articles, reviewed them using the predefined eligibility criteria. And resolved differences through consensus and the third author resolved any disagreements.
Quality assessment and data extraction
The quality of studies was also independently assessed by the two reviewers who used the recommendations indicated in the Cochrane Handbook (16). Six components were assessed: (I) adequate sequence generation; (II) allocation concealment; (III) blinding; (IV) incomplete outcome data; (V) free of selective reporting; and (VI) other bias.
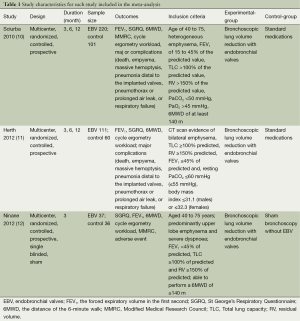
Data extraction and critical appraisal were carried out by the two reviewers independently. Any discrepancies were resolved by discussion or a third author, using a standardized data extraction spreadsheet, data on first author’s last name, the publication year, study design, the sample size, study population, inclusion criteria, treatment method, length of follow-up, and outcomes were extracted (Table 1).
Statistical analyses
We used RevMan (Version 5.2 Nordic Cochrane Centre) to analyze the collected data. Outcomes were pooled using RR for dichotomous variables and WMD for continuous variables with 95% CI. Heterogeneity was measured by the I2 test (17-19), with values of ≤25% absence, 26% to 39% unimportant, 40% to 60% moderate, and 60% to 100% substantial level of heterogeneity. Or set a P value <0.1 was considered statistically significant. When the hypothesis of homogeneity was not rejected, a fixed-effects model was used for the difference among patients with EBV and standard medications or sham EBV; otherwise, a random-effects model was used. We set statistical significance at a P value <0.05.
Results
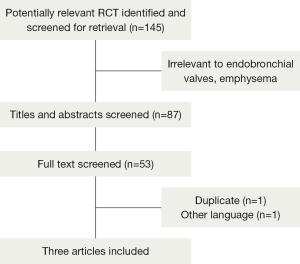
One hundred and forty-five articles were identified, after reading the titles and abstracts, 87 articles were excluded because they were abstracts, reviews, or animals experiments, or irrelevant to EBV, emphysema. After review of the full-text articles, 53 articles were excluded for non-controlled trials from the meta-analysis, and five articles remained. Two articles were excluded because one was duplicated data, the other was in French and incomplete data. At last only three trials (10-12) were included, with a total number of 565 randomized patients, of whom 368 received EBV and 161 received standard medications, 36 received sham bronchoscope. Table 1 shows the characteristics of the included studies, and the progress of searching is outlined in (Figure 1).Three RCT trials included 368 experiments and 197 controls in this meta-analysis, two trials compared with EBV and standard medications (10,11), and one compared with EBV and sham bronchoscope (12). All eligible studies were of high quality, and with a duration of more than 3 months follow up. Two included studies (10,11) referenced randomized, neither describe the specific method, no did reference the allocation concealment. One study (12) was single-blinded, and mentioned the method of randomization and allocation concealment, the other two (10,11) were non-blinded. Patients with standard medical treatments for emphysema (10,11), which include smoking cessation, bronchodilators, pulmonary rehabilitation programs, and long-term home oxygen therapy (20). The sham-trial (12) was a prospective, randomized, multicentre, single-blinded, sham-controlled study; patients underwent bronchoscopy and valve placement (treatment group) or a bronchoscopy without valve implantation (control group) and were followed for 3 months.
Full table
The primary outcome
Change in the percentage of FEV1 (FEV1%)
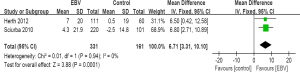
Two trials (N=492) were included (10,11). Overall, EBV lung volume reduction produced a significant increase in (mean change from baseline) FEV1% when compared to standard medications (WMD =6.71; 95% CI, 3.31 to 10.10; P=0.0001). Heterogeneity was not statistically significant (I2=0%; P=0.94) compare EBV with standard medications, so a fixed-effects model was calculated (Figure 2).
Secondary outcomes
Change in St George’s Respiratory Questionnaire (SGRQ) score
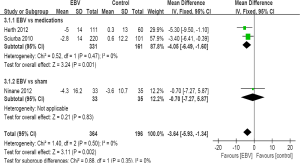
The pooled three results (565 participants) indicated that treatment with EBV could statistically change SGRQ score from baseline (WMD =−3.64; 95% CI, −5.93 to −1.34; P=0.002). Heterogeneity was not statistically significant (I2=0%; P=0.5) compared EBV with standard medications and sham EBV, a fixed-effects model was used (Figure 3). Among subgroups (10,11), the effect was also obvious. SGRQ score decreased significantly in trials between EBV and standard medications (WMD =−4.05; 95% CI, −6.49 to −1.60; P=0.001), and the two studies were homogeneous (I2=0%; P=0.47). Most importantly, the changes of SGRQ score in the subgroup were also higher than the minimal clinically important difference (MCID) of 4 points (21,22), and achieved the clinical effect.
Change in the Modified Medical Research Council (MMRC) dyspnoea score
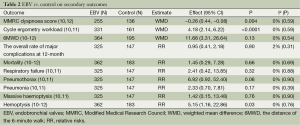
Two trials (10,12) reported the MMRC dyspnoea score, dyspnoea was measured by MMRC score, ranging from zero to four, with a higher score indicating more severe dyspnoea, MCID is one point (10). The score was statistically lower with EBV than with control in MMRC dyspnoea score (WMD =-0.26; 95% CI, -0.44 to -0.08; P=0.004), but didn’t achieve the MCID of one point in EBV. The trials were statistically homogeneous (I2=0%; P=0.59), a fixed-effect model was followed (Table 2).
Full table
Change in cycle ergometry workload
Two trials (10,11) showed data of the cycle ergometry workload, the results of each study suggested significant greater improvement in patients treated with EBV than with standard medications. The pooled analysis showed statistically significant improvement in cycle ergometry workload (WMD =4.18; 95% CI, 2.14 to 6.22; P<0.0001). The trials were homogeneous (I2=0%; P=0.59), a fixed-effect model was used (Table 2).
Change in the distance of the 6-minute walk (6MWD) test
The pooled three trials suggested the mean improvement in 6MWD test was failed to appear (WMD =11.66; 95% CI, −3.31 to 26.64; P=0.13, Table 2), the heterogeneity was not evident (I2=0%; P=0.54). The difference was also not found among subgroups.
Safety
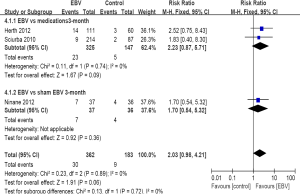
All three studies reported the major complications or adverse events, the overall rate of major complications didn’t differ compared EBV with standard medications and sham EBV at 3 months (RR =2.03; 95% CI, 0.98 to 4.21; P=0.06, Figure 4). Studies were highly homogeneous (I2=0%; P=0.89). The major complications consisted of death, empyema, massive hemoptysis, pneumonia distal to the implanted valves, pneumothorax or prolonged air leak, and respiratory failure. There were also no significant differences in the rate of major complications at 12 months between EBV and standard medications (RR =0.95; 95% CI, 0.41 to 2.18; P=0.9, Table 2).
Compare with standard medications and sham EBV (Table 2), the EBV didn’t increase the overall rate of morality (10-12) (RR =1.45; 95% CI, 0.29 to 7.28; P=0.66), respiratory failure (10,11) (RR =2.41; 95% CI, 0.42 to 13.85; P=0.32), pneumothorax or prolonged air leak (10,11) (RR =6.92; 95% CI, 0.92 to 52.40; P=0.06), pneumonia (10,11) (RR =2.33; 95% CI, 0.70 to 7.81; P=0.17), massive haemoptysis (10,11) (RR =1.42; 95% CI, 0.15 to 13.48; P=0.76), no empyema was found in all cases. However, there was an evident increase in haemoptysis associated with the EBV patients (10-12) (RR =5.15; 95% CI, 1.16 to 22.86; P=0.03) (Table 2).
Discussion
Currently, the most widely bronchoscopic lung volume reduction studied method to treat emphysema is EBV. Similar benefits with LVRS, minimally invasive endoscopic alternatives and less risk of associated complications (23). This meta-analysis shows that EBV therapy for the advanced emphysema with standard medications and sham EBV is associated with a statistically significant increase in lung function FEV1%, and health status SGRQ score, MMRC dyspnoea score, cycle ergometry workload, a similar effect of 6MWD without incurring a higher overall rate of major complications during 3 or 12 months. Compared with LVRS, the EBV therapy mimicking the physiological mechanism of LVRS, has the larger application, and fewer life-threatening complications. Our findings raise the possibility of therapy with the placement of EBV for advanced emphysema to alleviate symptoms.
In terms of FEV1%, both EBV treatments were better than standard medications (10,11) at 6 months. The sham trial reported FEV1 in liters, and non-significant improvement compared EBV and control in FEV1 at 3 months (12). For SGRQ score, it ranges from 0 to 100, with a higher score indicating a worse quality of life (10). Results suggested that the overall effect was significant; the mean change across all participants was close to the clinically significant change in SGRQ of four points. The patient in EBV treatment was clearly more marked than standard medications at 6 months, results for sham EBV were comparable to EBV therapy at 3 months for SGRQ score. Changes in MMRC dyspnoea score and cycle ergometry workload also appeared significant. Compared with EBV and control, the mean change in the MMRC across all participants was low than the MCID of one point. We didn’t find a significant clinical meaning in 6MWD test, which indicated EBV treatment provided a comparable level of exercise tolerability to standard medications and sham EBV.
More recently trials indicated that patients who undergo the placement of EBV developed atelectasis had greater benefits in terms of lung function, exercise capacity and a prolonged survival (24), there was also showed a more favourable result in patients treated with EBV who presented with complete fissures between the treated and adjacent lobes. Patients with intact interlobar fissure suggested lacking of collateral ventilation and better effect, the existence of collateral ventilation prevented majority of patients from the atelectasis (25,26). Measurements of collateral ventilation using the Chartis system which is a catheter-based system that measures pressures and flows during respiration and calculates the resistance of the collateral channel (27).
Based on the results of the safety evaluations, EBV therapy was generally well tolerated, the rate of major complications was similar compared EBV with standard medications and sham EBV at 3 or 12 months. Complications included mortality, respiratory failure, empyema, pneumonia, pneumothrax were non-significantly increased among those randomized to EBV patients compared with standard medications or sham EBV. Our results showed that only haemoptysis was increased caused by the EBV treatment, this is an expected adverse event following a thoracic procedure. Although it is a minimally invasive therapy, that may damage the bronchial wall and small vascular, and induced haemoptysis. However, the massive haemoptysis and hypovolemic shock rarely occur. Hemoptysis is often self-limiting and massive hemoptysis means resulting in respiratory failure or blood loss >300 mL in 24 h. Even some studies reported the common complications after EBV treatment, most of them didn’t reach an obvious clinical meaning level, and could be resolved medically using conventional therapies.
Consistent with the previous studies about earlier uncontrolled that demonstrated bronchial valve therapy of emphysema has acceptable safety and effectiveness (14,15,28). The bronchoscopic lung volume reduction with EBV is designed to induce lobar atelectasis by blocking airflow enter into the most emphysematous areas of lung, allowing expiration and the drainage of bronchial secretions, represents an example of new bronchoscopic treatment approach developed to obtain a reliable palliation of symptoms, less invasive, reversible and safer lung volume reduction (29).
Bronchoscopic lung volume reduction with EBV is an innovative intervention for advanced emphysema that targets the isolated emphysematous segment and results in a prolonged improvement, a single-center group assessed 18 patients at 3 years and 9 at 5 years confirmed our short-term encouraging results (30). However, because it is a novel experimental treatment, only three RCTs have been published to date (10-12). By pooling these data, the present meta-analysis indicated that EBV therapy provided clinically significant and promising improvements in patients with advanced emphysema.
Some limitations to the present meta-analysis are as follows. Firstly, the data is scarce, the sample size is not large enough to provide decisional clinical evidence, and long-term efficacy and safety with valve placement are poorly understood because follow-up in most studies is limited to 12 months. Secondly, potential prognostic, determined factors included heterogeneity, complete fissure, complete occlusion demonstrated in some trials (10,11,25,30,31), in this meta-analysis was not possible to pool an analysis. Finally, unpublished studies or other non-English languages studies with insufficient information or with null results were not included, which may have biased our results.
We need to provide more data, further RCT studies should be focused on larger sample size, long-term consequences with the placement of EBV, and studies conducted to determine which group of emphysema would achieve the optimal clinical benefits from the bronchoscopic lung volume reduction with EBV. And the forthcoming randomized, double-blind placebo-controlled trial of EBV placement for patients with high heterogeneity and intact fissures in COPD will prove this (32).
In conclusion, this meta-analysis clearly demonstrated that compared with medications or sham EBV treatment, EBV significantly increased lung function, health status and was generally well tolerated and safe in patients with advanced upper lobe-predominant emphysema. Additional clinical trials are needed to confirm which groups will benefit most from the bronchoscopic lung volume reduction with EBV in the long run.
Acknowledgements
Conceived and designed the experiments: LH, XM; Performed the experiments: NSS, LH; Analyzed the data: XYQ, GJ; Contributed analysis tools: NSS, XYQ; Wrote the paper: LH, XM;
This study was supported by Natural Science Foundation of China (NO. 30971306); Jiangsu Province “333 high-level personnel training project”; Six big talent peak in Jiangsu province project (the seventh batch NO: 033); Nantong social development project (NO: S2009023; NO: S2008017); and Nantong fourth period “226 high-level personnel training project” project and Rugao science and technology development project (No: 2013-72-68).
Disclosure: The authors declare no conflict of interest.
References
- Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008;300:2407-16. [PubMed]
- Rodrigo GJ, Castro-Rodriguez JA, Plaza V. Safety and efficacy of combined long-acting beta-agonists and inhaled corticosteroids vs long-acting beta-agonists monotherapy for stable COPD: a systematic review. Chest 2009;136:1029-38. [PubMed]
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543-54. [PubMed]
- Criner GJ, Cordova FC, Furukawa S, et al. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:2018-27. [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [PubMed]
- Naunheim KS, Wood DE, Krasna MJ, et al. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg 2006;131:43-53. [PubMed]
- Herth FJ, Gompelmann D, Ernst A, et al. Endoscopic lung volume reduction. Respiration 2010;79:5-13. [PubMed]
- Cohen E. Bronchoscopic treatment of end-stage chronic obstructive pulmonary disease. Curr Opin Anaesthesiol 2014;27:36-43. [PubMed]
- Delage A, Marquette CH. Bronchoscopic treatments for emphysema. Rev Mal Respir 2011;28:e108-14. [PubMed]
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [PubMed]
- Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334-42. [PubMed]
- Ninane V, Geltner C, Bezzi M, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J 2012;39:1319-25. [PubMed]
- Yim AP, Hwong TM, Lee TW, et al. Early results of endoscopic lung volume reduction for emphysema. J Thorac Cardiovasc Surg 2004;127:1564-73. [PubMed]
- Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest 2006;129:518-26. [PubMed]
- Sterman DH, Mehta AC, Wood DE, et al. A multicenter pilot study of a bronchial valve for the treatment of severe emphysema. Respiration 2010;79:222-33. [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589-624. [PubMed]
- Strange C, Herth FJ, Kovitz KL, et al. Design of the Endobronchial Valve for Emphysema Palliation Trial (VENT): a non-surgical method of lung volume reduction. BMC Pulm Med 2007;7:10. [PubMed]
- Troosters T, Casaburi R, Gosselink R, et al. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:19-38. [PubMed]
- Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD 2005;2:75-9. [PubMed]
- Venuta F, de Giacomo T, Rendina EA, et al. Bronchoscopic lung-volume reduction with one-way valves in patients with heterogenous emphysema. Ann Thorac Surg 2005;79:411-6. [PubMed]
- Hopkinson NS, Kemp SV, Toma TP, et al. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur Respir J 2011;37:1346-51. [PubMed]
- Gompelmann D, Eberhardt R, Michaud G, et al. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a feasibility study. Respiration 2010;80:419-25. [PubMed]
- Fessler HE. Collateral ventilation, the bane of bronchoscopic volume reduction. Am J Respir Crit Care Med 2005;171:423-4. [PubMed]
- truba J, Collins J, Herth FJ. Successful treatment of ventilator dependent emphysema with Chartis treatment planning and endobronchial valves. Int J Surg Case Rep 2011;2:285-7. [PubMed]
- Wood DE, McKenna RJ Jr, Yusen RD, et al. A multicenter trial of an intrabronchial valve for treatment of severe emphysema. J Thorac Cardiovasc Surg 2007;133:65-73. [PubMed]
- Galluccio G, Lucantoni G. Bronchoscopic lung volume reduction for pulmonary emphysema: preliminary experience with a new NOVATECH endobronchial silicone one-way valve. Interact Cardiovasc Thorac Surg 2010;11:213-5. [PubMed]
- Venuta F, Anile M, Diso D, et al. Long-term follow-up after bronchoscopic lung volume reduction in patients with emphysema. Eur Respir J 2012;39:1084-9. [PubMed]
- Eberhardt R, Gompelmann D, Schuhmann M, et al. Complete unilateral vs partial bilateral endoscopic lung volume reduction in patients with bilateral lung emphysema. Chest 2012;142:900-8. [PubMed]
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (The BeLieVeR-HIFi trial): study design and rationale. Thorax 2015;70:288-90. [PubMed]