PaO2 greater than 300 mmHg promotes an inflammatory response during extracorporeal circulation in a rat extracorporeal membrane oxygenation model
Introduce
The use of extracorporeal circulation (ECC), such as extracorporeal membrane oxygenation (ECMO), can be life-saving because it provides an appropriate oxygen delivery and blood flow rate to principal organs (1). ECMO is being increasingly used for mechanical support of respiratory and cardio-circulatory failure. An excessive systemic inflammatory response with similar clinical features is observed during sepsis and after cardiopulmonary bypass (CPB) (2). During ECMO therapy, activation of complement and contact systems occurs, which may be followed by cytokine release (3).
There are several factors that appear to be cause for the systemic inflammatory reaction, such as contact of blood with the ECC device’s surface, surgical operation trauma, endotoxemia, blood loss, and ischemic reperfusion injury (4). The inflammatory response that occurs during ECC is aggravated by the increase in cytokines, such as necrosis factor, interleukins and bradykinin (5), that occurs (6). In a recent study using a rat model, we showed that ECC results in a systemic inflammatory response with organ damage (7-11).
Furthermore, a recent systematic review meta-analysis and cohort study showed that, in patients resuscitated from cardiac arrest and admitted to the intensive care unit, significantly higher in-hospital mortality was seen in the hyperoxia condition management group than in the normoxia condition management group (12,13). A previous study showed that oxidative cell damage is produced by hyperoxia through the reactive oxygen species (ROS) production and inflammatory cytokine secretion (14). However, during clinical ECMO currently, the partial pressure of arterial oxygen (PaO2) is maintained at very high levels (15). In addition, there are reports of adverse effects in pediatric cardiac patients during high-oxygen management ECMO (15). However, the PaO2 that enhances the inflammatory response was unclear in previous studies.
Therefore, the subjects in the present study were divided into groups according to the PaO2 during ECMO, and the effects of PaO2 levels on serum cytokine [tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-10 (IL-10)] levels were investigated in a rat ECMO model. Additionally, the lung wet-to-dry weight (W/D) ratio was measured and used as an indication of lung tissue edema. Finally, generation of superoxide in the lung and liver tissues was detected by dihydroethidium (DHE) staining.
Methods
Animals
This study was conducted with the approval of National Cerebral and Cardiovascular Center Research Institute Animal Care and Use Committee and the Niigata University of Health and Welfare Animal Care and Use Committee. All procedures were performed in accordance with the National Institutes of Health guidelines for animal care. The subjects were male Sprague-Dawley (SD) rats (400–450 g) that were housed three per cage under a 12-hour light-dark cycle with food and water available ad libitum. The SD rats were purchased from Japan SLC Inc (Shizuoka, Japan)
Anesthesia, surgical preparation, and ECMO
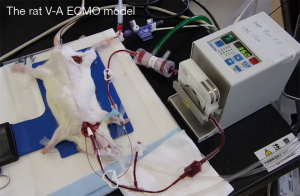
After the animals were anesthetized with 5.0% isoflurane mixed oxygen enriched air inhalation with a vaporizer, they were placed in the supine position, and a rectal temperature probe was then inserted. Following orotracheal intubation using a 14-G catheter (Terumo Corp, Tokyo, Japan), the animals were mechanically ventilated under 40% of oxygen fraction with a Model 687 respirator (Harvard Apparatus Ltd., Edenbridge, Kent, UK) providing volume-controlled ventilation at a frequency of 70/min, with tidal volume of 8–10 mL/kg body weight. Isoflurane 2.0–2.5% was used to maintain anesthesia, and the rectal temperature was kept at 35–36 °C. The right femoral artery was cannulated with SP-31 polyethylene tubing (Natsume Seisakusho Co., Ltd, Tokyo, Japan) for arterial blood pressure monitoring using a Power-Lab system (Model ML870, AD Instruments Japan Inc., Nagoya, Japan). SP-55 polyethylene tubing (Natsume Seisakusho Co., Ltd) was used to cannulate the left common carotid artery as the arterial return cannula for the ECMO system, and heparin sodium (500 IU/kg) was given through this cannula. A 16G cannula (Togo-medkit Co., Ltd, Tokyo, Japan) was passed through the right internal jugular vein advanced into the right atrium as the conduit for venous uptake. The ECMO system consisted of a roller pump (REGLO Digital MS-2/6, ISMATEC, Wertheim, Germany), a miniature membrane oxygenator (Senko Medical Instrument Mfg. Co., Ltd, Tokyo, Japan), and polyvinyl chloride tubing line (Senko Medical Instrument Mfg. Co., Ltd). ECMO circuit was primed by 7.5 mL of Ringer’s solution with 0.5 mL (500 IU) of heparin. Figure 1 shows the experimental conditions.
Experimental design
The animals randomly assigned to one of the following groups depending on the value of PaO2 during ECMO: A group (n=11), with PaO2 maintained at 100–199 mmHg; B group (n=10), with PaO2 maintained at 200–299 mmHg; C group (n=8), with PaO2 maintained at 300–399 mmHg; and D group (n=11), with PaO2 maintained at greater than 400 mmHg during ECMO. In all experiment, normothermic ECMO with a pump flow of 70 mL/kg/min. During the experimental period, the partial pressure of arterial carbon dioxide (PaCO2) was ordinarily maintained at 30–40 mmHg in all groups.
Arterial blood samples were collected at three defined time points: before ECMO (pre-ECMO); 60 min after initiation of ECMO; and 120 min after initiation of ECMO (end-ECMO).
TNF-α, IL-6, and IL-10 levels were measured by multiplex suspension array (Bio-PlexTM Assay Kits, Hercules, CA, USA) estimation of systemic inflammatory responses. Blood gases, pH, hemoglobin (Hb) concentration, and electrolytes were also measured (VetStat Electrolyte and Blood Gas Analyzer, IDEXX, New South Wales, Australia). All animals were sacrificed at the end of ECMO by potassium chloride injection into the heart, and the left lung was harvested and divided into three parts. The superior third was used for calculation of the W/D ratio. The lung block was weighed before and after desiccation for 48 h in a dry oven at 70 °C. Additionally, the right lung and part of the liver were placed in cold PBS buffer and then embedded in dry ice acetone for frozen section. The frozen segments were cut into 7-µm-thick transverse sections that were then placed on glass slides. DHE stain solution (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) diluted with dimethyl sulfoxide thirty thousand times was applied topically to each tissue section. The slides were incubated in a light-protected chamber at 37 °C for 30 min. Images of the tissue sections were obtained using a fluorescence microscope (exposure time 80 ms, red fluorescence, 594 nm) with a rhodamine filter. Fluorescence intensity, which correlates positively with the amount of superoxide generation, was determined in the lung and liver tissues using image processing software (Imag v1.60, National Institutes of Health, Bethesda, MD).
Statistics
All value is presented as means ± standard error (SE). Comparisons among groups were performed by analysis of variance (ANOVA). The Fisher Protected Least Significant Difference (PLSD) post hoc test was used for subsequent comparisons between groups at the same point in time. Statistical analyses were performed with Stat View 5.0 (Abacus Concepts, Berkeley, CA, USA). Statistical significance was assumed when the P value was less than 0.05.
Results
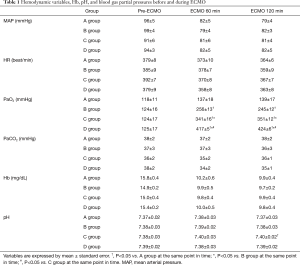
Table 1 shows the changes in hemodynamic variables, Hb concentration, pH, and PaO2 and PaCO2 in the A, B, C, and D groups during the experiments. Hemodynamics was stable during experiment, there was no need for administration of catecholamine. Before ECMO, the serum levels of inflammatory cytokines were not significantly different among the A, B, C, and D groups. The PaO2 level was 137±18 mmHg in the A group, 256±13 mmHg in the B group, 341±16 mmHg in the C group, and 417±5 mmHg in the D group during ECMO, while no significant difference was found in the PaCO2 levels among these groups.
Full table
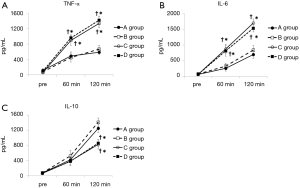
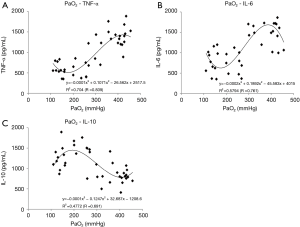
In the D group, TNF-α increased significantly, reaching a maximum (1,419±109 pg/mL), and in the C group, IL-6 increased significantly, reaching a maximum (1,700±132 pg/mL) at the end of ECMO. In the A and B groups, though, the increases in pro-inflammatory cytokines (TNF-α and IL-6) were significantly suppressed by approximately 60% compared to the C and D groups (Figure 2A,B). On the other hand, in the B group, the anti-inflammatory cytokine (IL-10) increased significantly, reaching a maximum (IL-10: 1,412±111 pg/mL) at the end of ECMO, approximately 70% higher as compared to the C and D groups at the same point in time (Figure 2C). The relationships between oxygen partial pressure during ECMO and cytokine levels (plotted display and approximate curve) are shown in Figure 3.
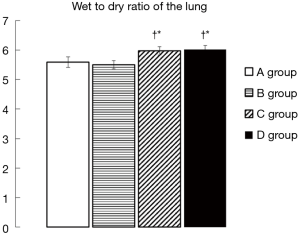
The C and D groups showed significantly higher W/D ratios than the A and B groups (A group 5.59±0.18, B group 5.50±0.14, C group 5.98±0.14, D group 6.02±0.13) (Figure 4). However, the increases in the W/D ratio were significantly suppressed in the A and B groups compared to the C and D groups.
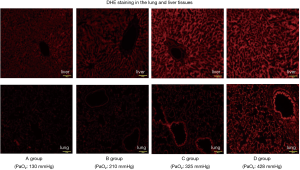
DHE staining in the lung and liver tissues was markedly enhanced in the C and D groups compared to the A and B groups (Figures 5,6), indicating greater superoxide production with a hyperoxia condition management during ECMO.

Discussion
This is the first study to show that the pro-inflammatory cytokines increased significantly more in the hyperoxia ECMO situation, while the anti-inflammatory cytokine surge was more suppressed in the C and D (PaO2 ≥300 mmHg) groups than in the A and B (PaO2 <300 mmHg) groups. Higher W/D ratios at the end of ECMO were seen in the lung tissues of rats in the C and D groups than in the A and B groups, suggesting that they accumulated more water. Additionally, based on DHE staining for superoxide production, there appeared to be a marked increase in the lung and liver tissues in the C and D groups, but there was none in the A and B groups. In the present study, serum levels of the cytokines TNF-α, IL-6, and IL-10 were significantly increased during ECMO, indicating the presence of a systemic inflammatory response and organ damage in this rat ECMO model. Furthermore, during ECMO, arterial blood pressure was maintained at around 80 mmHg, and Hb was maintained at around 10 g/dL. These data suggest that the present rat ECMO model corresponds to the established human ECMO procedure, which is often accompanied by systemic inflammation and organ damage (2,3).
Several factors may be responsible for the systemic inflammatory response during ECMO, including contact of the blood with the ECMO unit surface, surgical trauma, endotoxemia, blood loss, and ischemic reperfusion injury (4). Several studies have shown that leucocytes, platelets, and the complement system are activated by the walls of the ECC device. As a result, the activated leukocytes release cytotoxic agents and ROS that are associated with the systemic inflammatory response and organ damage (16,17). This additional increase in cytokines, especially pro-inflammatory cytokines (5), heightens the inflammatory response (6). Thus, further inflammation is caused by these complex interactions between the surgical procedure and ECC (6).
We hypothesized that hyperoxia condition stimulate generation of ROS, heighten the systemic inflammatory response, and lead to organ tissue damage during ECMO. The superoxide anion is the primary product of cellular ROS production. As this study showed, intracellular superoxide production could be detected by DHE stain fluorescence, which is a widely used technique in which superoxide reacts with the hydroethidine moiety of DHE to produce ethidium (18). In the present study, DHE stain fluorescence (Figures 5,6) of the lung and liver tissues was noticeably enhanced in the hyperoxia condition ECMO (PaO2 ≥300 mmHg) group, indicating more superoxide production during ECMO with hyperoxia condition. This implies that, given the widespread increase in ROS generation and the increased levels of organ damage markers, the hyperoxia condition ECMO caused direct oxidative cellular harm. Related to this, we previously found that hydrogen gas administration through removal of the hydroxyl radical (the most cytotoxic radical) suppressed the increased levels of organ damage biomarker during ECC in the rat (7). Given the relationship between PaO2 during ECMO and cytokine levels (plotted display and approximate curve: Figure 3A,B), sharp pro-inflammatory cytokine (TNF-α, IL-6) level increases were seen at PaO2 levels of 356 and 315 mmHg, respectively. This inflection point shows that we can minimize elevation of pro-inflammatory cytokines by controlling PaO2 to <300 mmHg.
Since T lymphocyte activation and cytokine production by ROS occur via redox-sensitive signal pathways (19), the hyperoxia condition may also have increased cytokine expression through stimulate generation of ROS in the current rat ECMO model. The present study showed not only that serum pro-inflammatory cytokine levels and organ damage were significantly increased by hyperoxia (PaO2 ≥300 mmHg) in the C and D groups during ECMO, but that the hyperoxia also resulted in significant suppression of the expression of the anti-inflammatory cytokine (IL-10) compared to PaO2 <300 mmHg in the A and B groups. This suppression is thought to augment the inflammatory responses in the PaO2 ≥300 mmHg group, because IL-10 regulates pro-inflammatory cytokine production (20). Therefore, based on the present findings, appropriate oxygen control (<300 mmHg) not only attenuates pro-inflammatory cytokine production, but it also increases anti-inflammatory cytokine production, which decreases inflammatory responses during ECMO in the rat.
In our previous study, selective reduction of hydroxyl radicals with hydrogen gas was shown to attenuate both pro- and anti-inflammatory cytokines, which implies that this radical non-selectively increases these cytokines (7). However, the mechanism for the increase in pro-inflammatory cytokines but the decrease in the anti-inflammatory cytokine under increased superoxide production seen in the present study is unclear. There is a possibility that hyperoxia condition affects cytokine specific reactions through a mechanism other than the ROS generation pathway. It should be noted, on the other hand, it has been reported that hyperoxia condition down regulated the IL-10 gene in fetus rat alveolar type II cells in cell culture (21).
In the present study, there was a sharp increase in the lung W/D ratio at PaO2 ≥300 mmHg. This increase in the W/D ratio of the lung implies the development of pulmonary edema during ECMO, consistent with a previous report (22) and our earlier rat ECC model study (7). A new finding of the current study is that avoiding oxygen oversupply suppressed the increase in the W/D ratio ECMO. Since the pulmonary capillary endothelial cells show the primary symptom of hyperoxic lung cell injury in hyperoxic condition (23,24), maintenance of normoxia may attenuate vascular endothelial injury through suppression of the production of superoxide and pro-inflammatory cytokines during ECMO. Notably, one human study showed that hyperoxic exposure alters alveolar capillary permeability with secretion of mediators by alveolar macrophages (25). In the present study, the W/D ratios suggest that excessive hyperoxic condition increased pulmonary vascular permeability. In addition, there have been many reports of hyperoxia-induced lung injury, and the treatment and remedial measures for such injury have been studied (25,26). Furthermore, oxygen toxicity is known to be strongly dependent on both PaO2 and exposure time. It is fair to assume that the inflammatory response will increase with time even with lower PaO2 levels. ECMO exposure time will be a focus of subsequent research.
Our plan is to further study the damage caused by hyperoxia during ECMO, from which we hope to elucidate the mechanism and then propose the appropriate PaO2 for ECMO management.
Limitations
This study has several limitations. First, hyperoxic ECMO appeared to aggravate the inflammatory response and tissue injury based on the changes in the levels of serum cytokines and W/D ratio of the lung. On the other hand, histologic analyses of cellular injury were limited to the lung and liver. The additional research about other organs, especially principal organs such as the kidney, brain and heart, are needed to verify this. Second, it was not possible to set up a ventilator according to the rules of protective lung ventilation and considering the guidelines. In the future, it is necessary to consider the construction of the veno-venous ECMO model and the respiratory setting. Finally, the present rat model of ECMO appears to be equivalent to the established ECMO procedure for human patient. Although, there is a need to study the effect of the PaO2 level on the systemic inflammatory responses and organ damage during ECMO in larger animal, long-term ECMO assist models before we can consider applying these findings to clinical site.
Conclusions
In conclusion, based on the results of the present study, the systemic inflammatory response and organ damage including pulmonary edema occurred along with cytokine and superoxide production in the rat ECMO model. Hyperoxia (PaO2 ≥300 mmHg) intensifies these responses, while pro-inflammatory cytokine production is enhanced and anti-inflammatory cytokine production is attenuated. In our view, it is important to maintain appropriate oxygen levels (not hyperoxia) in order to minimize the systemic inflammation and lung injury during ECMO. Furthermore, this rat ECMO model appears to be equivalent to the established ECMO for human patient procedure and is thus useful for investigating the pathophysiological changes that occur during artificial perfusion.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form and declare: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted with the approval of National Cerebral and Cardiovascular Center Research Institute Animal Care and Use Committee and the Niigata University of Health and Welfare Animal Care and Use Committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Walker G, Liddell M, Davis C. Extracorporeal life support-state of the art. Paediatr Respir Rev 2003;4:147-52. [Crossref] [PubMed]
- Träger K, Fritzler D, Fischer G, et al. Treatment of post cardiopulmonary bypass SIRS by hemoadsorption: a case series. Int J Artif Organs 2016;39:141-6. [Crossref] [PubMed]
- Millar JE, Fanning JP, McDonald CI, et al. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care 2016;20:387. [Crossref] [PubMed]
- Baehner T, Boehm O, Probst C, et al. Cardiopulmonary bypass in cardiac surgery. Anaesthesist 2012;61:846-56. [Crossref] [PubMed]
- Ben-Abraham R, Weinbroum AA, Dekel B, et al. Chemokines and the inflammatory response following cardiopulmonary bypass--a new target for therapeutic intervention?--A review. Paediatr Anaesth 2003;13:655-61. [Crossref] [PubMed]
- Liguori GR, Kanas AF, Moreira LF. Managing the inflammatory response after cardiopulmonary bypass: review of the studies in animal models. Rev Bras Cir Cardiovasc 2014;29:93-102. [Crossref] [PubMed]
- Fujii Y, Shirai M, Inamori S, et al. Insufflation of Hydrogen Gas Restrains the Inflammatory Response of Cardiopulmonary Bypass in a Rat Model. Artif Organs 2013;37:136-41. [Crossref] [PubMed]
- Fujii Y, Shirai M, Tsuchimochi H, et al. Hyperoxic condition promotes an inflammatory response during cardiopulmonary bypass in a rat model. Artificial Organs 2013;37:1034-40. [Crossref] [PubMed]
- Fujii Y, Shirai M, Pearson JT, et al. Changes in inflammatory response during and after cardiopulmonary bypass using a rat extracorporeal circulation model. Conf Proc IEEE Eng Med Biol Soc 2015;2015:957-60.
- Fujii Y, Tanabe T, Yamashiro T, et al. Hydroxyethyl Starch Priming on the Systemic Inflammatory Response and Lung Edema after Cardiopulmonary Bypass in a Rat Model. ASAIO J 2017;63:618-23. [Crossref] [PubMed]
- Sukumaran V, Tsuchimochi H, Fujii Y, et al. Ghrelin Pre-treatment Attenuates Local Oxidative Stress and End Organ Damage During Cardiopulmonary Bypass in Anesthetized Rats. Front Physiol 2018;9:196. [Crossref] [PubMed]
- Damiani E, Adrario E, Girardis M, et al. Abele Donati. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care 2014;18:711. [Crossref] [PubMed]
- Page D, Ablordeppey E, Wessman BT., et al. Emergency department hyperoxia is associated with increased mortality in mechanically ventilated patients: a chort study. Crit Care 2018;22:9. [Crossref] [PubMed]
- Mittal M, Siddiqui MR, Tran K, et al. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid Redox Signal 2014;20:1126-67. [Crossref] [PubMed]
- Sznycer-Taub NR, Lowery R, Yu S, et al. Hyperoxia Is Associated With Poor Outcomes in Pediatric Cardiac Patients Supported on Venoarterial Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med 2016;17:350-8. [Crossref] [PubMed]
- Goudeau JJ, Clermont G, Guillery O, et al. In high-risk patients, combination of antiinflammatory procedures during cardiopulmonary bypass can reduce incidences of inflammation and oxidative stress. J Cardiovasc Pharmacol 2007;49:39-45. [Crossref] [PubMed]
- Clermont G, Vergely C, Jazayeri S, et al. Systemic free radical activation is a Major event involved in myocardial oxidative stress related to cardiopulmonary bypass. Anesthesiology 2002;96:80-7. [Crossref] [PubMed]
- Suwanpradid J, Rojas M, Behzadian MA, et al. Arginase 2 deficiency prevents oxidative stress and limits hyperoxia-induced retinal vascular degeneration. PLoS One 2014;9:e110604. [Crossref] [PubMed]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47-95. [Crossref] [PubMed]
- Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest 2000;117:1162-72. [Crossref] [PubMed]
- Lee HS, Kim CK. Effect of recombinant IL-10 on cultured fetal rat alveolar type II cells exposed to 65%-hyperoxia. Respir Res 2011;12:68. [Crossref] [PubMed]
- Aebert H, Kirchner S, Keyser A, et al. Endothelial apoptosis is induced by serum of patients after cardiopulmonary bypass. Eur J Cardiothorac Surg 2000;18:589-93. [Crossref] [PubMed]
- Harijith A, Pendyala S, Ebenezer DL, et al. Hyperoxia-induced p47phox activation and ROS generation is mediated through S1P transporter Spns2, and S1P/S1P1&2 signaling axis in lung endothelium. Am J Physiol Lung Cell Mol Physiol 2016;311:L337-51. [Crossref] [PubMed]
- Wang J, Dong W. Oxidative stress and bronchopulmonary dysplasia. Gene 2018;678:177-83. [Crossref] [PubMed]
- Davis WB, Rennard SI, Bitterman PB, et al. Pulmonary oxygen toxicity Early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med 1983;309:878-83. [Crossref] [PubMed]
- Zhang Q, Wu D, Yang Y, et al. Dexmedetomidine Alleviates Hyperoxia-Induced Acute Lung Injury via Inhibiting NLRP3 Inflammasome Activation. Cell Physiol Biochem 2017;42:1907-19. [Crossref] [PubMed]