
Feasibility of electromagnetic navigation bronchoscopy-guided lung resection for pulmonary ground-glass opacity nodules
Introduction
Recent advances in imaging modalities have made it easier to diagnose lung cancer, and there has been an increase in the number of early lung cancer cases diagnosed using the recommended computed tomography (CT) program (1,2). In particular, many guidelines have suggested different methods to diagnose and treat small ground-glass nodules (GGNs) (3,4). Accurate histologic diagnosis of these lung lesions might enable appropriate surgical treatment.
Percutaneous core needle biopsy (PCNB) and conventional transbronchial biopsy are standard diagnostic procedures with high diagnostic yield for lung nodules. However, in the case of a small lung lesion, the diagnostic yield of PCNB is poor, and there is a relatively high rate of complications such as pneumothorax or hemothorax; moreover, radiation exposure is inevitable (5-7).
The accuracy rate of PCNB diagnosis is less than 67% in <1-cm-sized lung nodules despite being solid nodules (8). Moreover, the role of PCNB is controversial in the diagnosis of GGN lesions, with no consensus on the optimal size threshold (8).
In the case of small non-palpable GGNs, if sublobar resection is possible through accurate localization, the optimum advantage of the resection method, particularly the removal of the lesion with preservation of pulmonary function, can be guaranteed (2,9). Usually, GGNs are difficult to palpate using the fingers or an instrument, and are not visible with optical vision during video-assisted thoracoscopic surgery (VATS). Hence, it is very difficult to find the target lesion without localization during surgical resection. For this reason, various localization procedures have been proposed in the surgical resection of small lung nodules (10-13) including electromagnetic navigation bronchoscopy (ENB). Several studies have reported the efficacy of ENB procedures and, in most cases, have shown their higher accuracy and a lower incidence of complications than the conventional methods (14-20) of lung nodule resection. However, there is a lack of studies on ENB-guided surgical procedures for GGNs. Therefore, we evaluated the accuracy of ENB procedures targeting small GGNs and the effectiveness of surgical resection.
Methods
Patients
This study included patients who underwent ENB-guided lung resection for suspiciously malignant nodules at Chungnam National University Hospital from January 2017 to April 2019. Medical records were retrospectively reviewed.
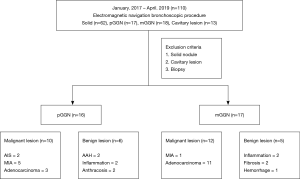
A multidisciplinary team, including a pulmonologist, radiologist, interventional radiologist, and surgeon, evaluated all the lesions before the ENB procedure. In total, 110 target lesions were surgically resected after tattooing or transbronchial biopsy. There were 35 cases of GGN lesions in 31 patients, and solid lesions and cavitary lesions were excluded from the analysis. A total of 33 lesions in 29 patients were included in this study, after excluding 2 transbronchial biopsy-only cases (Figure 1). The Institutional Review Board of the Chungnam National University Hospital approved this retrospective study.
3D reconstruction of virtual route and ENB-guided localization
To reconstruct the virtual route for navigation to the target lesion, the patients underwent chest CT with 1-mm cuts according to the manufacturer’s recommendations (Medtronic, Minneapolis, MN, USA) prior to surgery. Using the software provided by the manufacturer, we reconstructed the virtual 3D route to the target lesion by automatic and manual methods. We typically reconstructed several other routes (2 or 3 routes) in cases wherein the locatable guide was not able to follow the route.
All ENB procedures were performed directly by the thoracic surgeons in the operating room using the SuperDimension™ navigation system (Medtronic, Minneapolis, MN, USA) after general anesthesia induction using a single-lumen endotracheal tube (7.5 or 8.0 Fr).
If the locatable guide had reached the target lesion according to the actual bronchoscopic view and virtual route, 0.3 to 0.5 mL of indigo carmine was injected for marking. If the locatable guide could not access the target lesion, we followed another route for accurate marking. In cases that were inaccessible through all possible routes, we injected the dye at the closest point to the target lesion and considered the distance between the injection point and the target lesion on the axial, coronal, and sagittal CT images.
Surgical procedure
After the ENB procedure, the endotracheal tube was replaced with a double-lumen tube for surgery. Resection was performed using single-port VATS or the conventional three-port VATS. Single-port VATS was performed in specific cases of relatively peripherally located and small-sized nodules, and the tower crane technique was used (21). The target lesion was resected using a linear stapler guided by the marking imprinted by the indigo carmine dye. Surgical resection margins were judged grossly, and if the judgment was not clear, the surgical decision was determined through frozen section biopsy results. If there were invasive components in frozen section biopsy and further resection was required, anatomical resection such as segmentectomy or lobectomy with mediastinal lymph node dissection was performed.
Results
Patient and lesion characteristics
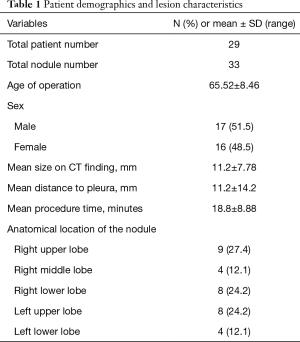
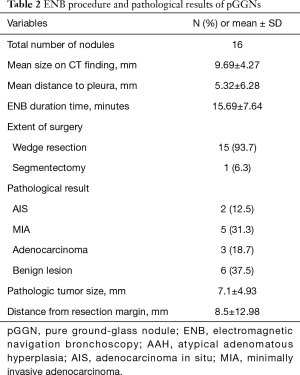
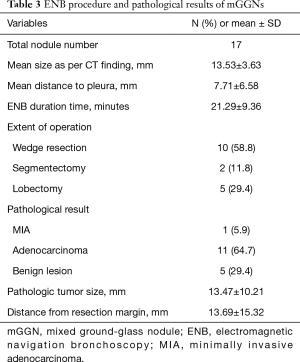
A total of 33 GGNs were localized by ENB-guided dye injection in 29 patients. Two patients each had 2 GGNs and one patient had 3 GGNs. There were 14 and 15 male and female patients, respectively, and the mean age was 65.52±8.46 years. Table 1 summarizes the clinical characteristics of the patients and lesions. Among the 33 GGNs, there were 16 pure GGNs (pGGNs) and 17 mixed GGNs (mGGNs) (Figure 2). The mean size of all lesions was 11.2±7.78 mm, and the mean distance to pleura was 11.2±14.2 mm. The mean ENB procedure time was 18.8±8.88 minutes. The lesions were found in 9 cases in the RUL, 4 cases in the RML, 8 cases in the RLL, 8 cases in the LUL, and 4 cases in the LLL. Tables 2 and 3 present the characteristics of pure and mixed GGNs in detail.
Full table
Full table
Full table
Operative results
All nodules were successfully resected by single-port VATS or the conventional three-port VATS without conversion to thoracotomy. We generally employed the conventional three-port VATS method, but if the target was located peripherally with no adhesion, or the location of the marked area could easily be visually confirmed at the operation field, we performed the resection by anchoring the suture through a single 15-mm port (tower crane technique) (21). VATS wedge resection was performed in most cases. However, if the location of the lesion made it difficult to secure a sufficient surgical resection margin with wedge resection, or if the lesion was located at the central portion, we performed segmentectomy with additional tattooing of the intersegmental plane by ENB.
In pGGNs, wedge resection was performed in 15 patients, and segmentectomy was performed in 1 patient. Three pGGNs were confirmed as invasive adenocarcinoma on the permanent pathological result, and the surgery was completed by lobectomy afterward.
In mGGO lesions, wedge resection was performed in 15 patients, and segmentectomy was performed in 2 patients. Conversion to lobectomy was required in 5 patients. In 6 patients with mGGNs with confirmed invasive adenocarcinoma by permanent pathology, we performed completion lobectomy. The overall postoperative course was uneventful. In two patients with noninvasive adenocarcinoma, resection was required to achieve sufficient safety margin.
Efficiency
In this study, ENB-guided target tattooing with indigo carmine injection for surgical resection of GGNs was successful in all cases. In all cases, proper tattooing was made possible by accessing the route set previously. Although the procedure time was relatively short, there was a tendency for the duration to be prolonged when the approach angle to the target was an acute angle, for example, when the lesion was situated in the anterior segment of both upper lobes.
Procedure accuracy and safety
We were able to obtain the accurate surgical specimens that were targeted preoperatively in all cases with sufficient pathological results. The final surgical pathology of the lung nodules revealed 22 malignancies in 33 lesions (66.7%). Of these, 19 and 3 were primary lung adenocarcinomas and metastatic lesions, respectively. Eleven nodules were diagnosed as benign, including atypical adenomatous hyperplasia, inflammation, and anthracofibrosis.
Tables 2 and 3 show the pathological results of the resected nodules in pGGO and mGGO lesions. The pathological results show 10 cases of malignant lesions and 6 cases of benign lesions among the pGGO lesions. In total, 12 malignant and 5 benign lesions were confirmed in mGGO lesions.
Among the 16 pGGO lesions, 10 cases were malignant. Two cases were adenocarcinomas in situ, 5 cases were minimally invasive adenocarcinomas (MIAs), and 3 cases were adenocarcinomas. Six benign lesions were identified as atypical adenomatous hyperplasia in 2 cases, anthracosis in 2 cases, and inflammation in 2 cases. The pathologic tumor size was 7.1±4.93 mm, and the distance from the resection margin was 8.5±12.98 mm.
In the 17 mGGO lesions, 12 cases showed adenocarcinoma. Of these 12 cases, one case was an MIA and 11 cases were adenocarcinomas. Five benign lesions were identified as inflammation in 2 cases and fibrosis in 3 cases. The pathologic tumor size was 13.47±10.21 mm, and the distance from the resection margin was 13.69±15.32 mm.
In all cases, there were no procedure-related complications or operative mortality.
Discussion
With the increase in routine health checkups with low-dose chest CT, we now have better chances in diagnosing GGNs. After acquiring pathological data, the highly suspected GGN lesions must be evaluated for not only differential diagnosis of lung cancer but also for definite treatment of early lung cancer (22). For the diagnosis of GGN lesions, PCNB is insufficient due to its high false-negative value (8). Thus, we need to consider surgical intervention in selective cases of GGNs.
ENB allows access to lesions that were not reachable with conventional bronchoscopy and lesions that were difficult to access by other percutaneous transthoracic approach methods with lower complications and higher accuracy rates and without radiation exposure. The localization of the target lesion in the operation field is one of the most important and difficult issues encountered particularly in small lung nodules.
There is no definite guideline for the localization of lung nodules during surgical resection. In the case of solid lesions, it is possible to perform resection without any localization procedures because they can be digitally palpated during operation even if their size is not large in the peripheral region. However, in the case of GGNs especially pGGNs, they cannot be palpated even if they are not relatively large.
Even for solid masses, Ciriaco et al. recommended the preoperative localization procedure for lung nodules <10 mm and those located >15 mm from the pleura (23). Nakashima et al. suggested that preoperative localization for lung lesion resection was required for cases that met two or more of the following criteria: (I) maximum diameter of the nodule of 5 mm or less; (II) maximum diameter to minimum distance between the visceral pleura and inferior border of the nodule of 5 mm or less, and (III) nodule with low densities (24).
Several conventional methods have been used to localize lung lesions prior to ENB. Localization of the wire guided by CT prior to surgery had a relatively high rate of wire dislodgment as well as complications such as pneumothorax and hemothorax, and radiation exposure is inevitable. In addition, other methods, such as fiducial placement, which was performed for localization through fluoroscopy in the operation field, have disadvantages in that they are associated with radiation exposure and difficult to perform because many centers do not have the capacity to adequately support these procedures in their facility.
Thus, recently, the transbronchial approach through ENB was widely performed for the localization of target lung lesions due to its many advantages compared to the conventional methods.
Previous studies have also documented the safety and efficacy of localization in the diagnosis and surgical resection of small peripheral pulmonary lesions by ENB, and the success rate was very high as well. Awais et al. reported a 100% success rate for ENB-guided dye marking for thoracoscopic resection of small pulmonary nodules (25). Marino et al. reported a 97.2% accuracy and two nodules that were localized by marking were identified by palpation (26). However, there is no study on ENB-guided localization for lung resection in GGNs. Therefore, in this study, we performed ENB-guided localization of relatively small GGNs (11.2±7.78 mm) withs high efficiency and accuracy.
One of the limitations of this study is that the selection bias could not be eliminated because of its retrospective study design and the single surgical team who performed the procedure. Moreover, we assessed a relatively few patients, and no comparison with the appropriate control group was made.
In conclusion, ENB is a feasible and highly accurate localization method for minimally invasive lung resection of small GGNs. Further studies on small GGO lesions should be performed in the future.
Acknowledgments
Funding: This work was supported by research fund of Chungnam National University.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.71). The authors have no conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work (if applied, including full data access, integrity of the data and the accuracy of the data analysis) in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Institutional Review Board of the Chungnam National University Hospital approved this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sihoe ADL, Cardillo G. Solitary pulmonary ground-glass opacity: is it time for new surgical guidelines? Eur J Cardiothorac Surg 2017;52:848-51. [Crossref] [PubMed]
- Sagawa M, Oizumi H, Suzuki H, et al. A prospective 5-year follow-up study after limited resection for lung cancer with ground-glass opacity. Eur J Cardiothorac Surg 2018;53:849-56. [Crossref] [PubMed]
- Dai J, Yu G, Yu J. Can CT imaging features of ground-glass opacity predict invasiveness? A meta-analysis. Thorac cancer 2018;9:452-8. [Crossref] [PubMed]
- Choi SH, Chae EJ, Shin SY, et al. Comparisons of clinical outcomes in patients with and without a preoperative tissue diagnosis in the persistent malignant-looking, ground-glass-opacity nodules. Medicine 2016;95:e4359. [Crossref] [PubMed]
- Lee HY, Lee IJ. Assessment of independent risk factors of developing pneumothorax during percutaneous core needle lung biopsy: focus on lesion depth. Iran J Radiol 2016;13:e30929. [Crossref] [PubMed]
- Jae LI, June IH, Miyeon Y, et al. Percutaneous core needle biopsy for small (</= 10 mm) lung nodules: accurate diagnosis and complication rates. Diagn Interv Radiol 2012;18:527-30. [PubMed]
- César DN, Torres US, D'Ippolito G, et al. CT-guided transthoracic core-needle biopsies of mediastinal and lung lesions in 235 consecutive patients: factors affecting the risks of complications and occurrence of a final diagnosis of malignancy. Arch Bronconeumol 2019;55:297-305. [PubMed]
- Tsukada H, Satou T, Iwashima A, et al. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol 2000;175:239-43. [Crossref] [PubMed]
- Sakurai H, Asamura H. Sublobar resection for early-stage lung cancer. Transl Lung Cancer Res 2014;3:164-72. [PubMed]
- Matsuura Y, Mun M, Ichinose J, et al. Recent fluorescence-based optical imaging for video-assisted thoracoscopic surgery segmentectomy. Ann Transl Med 2019;7:32. [Crossref] [PubMed]
- Tachihara M, Tamura D, Kiriu T, et al. Bronchoscopy using virtual navigation and endobronchial ultrasonography with a guide sheath (EBUS-GS) with or without fluoroscopy for peripheral pulmonary lesions. Kobe J Med Sci 2018;63:E99-104. [PubMed]
- Sobieszczyk MJ, Yuan Z, Li W, et al. Biopsy of peripheral lung nodules utilizing cone beam computer tomography with and without trans bronchial access tool: a retrospective analysis. J Thorac Dis 2018;10:5953-9. [Crossref] [PubMed]
- Huang HZ, Wang GZ, Xu LC, et al. CT-guided hookwire localization before video-assisted thoracoscopic surgery for solitary ground-glass opacity dominant pulmonary nodules: radiologic-pathologic analysis. Oncotarget 2017;8:108118-29. [Crossref] [PubMed]
- Khandhar SJ, Bowling MR, Flandes J, et al. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study. BMC Pulm Med 2017;17:59. [Crossref] [PubMed]
- Cho HJ, Roknuggaman M, Han WS, et al. Electromagnetic navigation bronchoscopy-Chungnam National University Hospital experience. J Thorac Dis 2018;10:S717-24. [Crossref] [PubMed]
- Hyun K, Park IK, Song JW, et al. Electromagnetic navigation bronchoscopic dye marking for localization of small subsolid nodules: retrospective observational study. Medicine 2019;98:e14831. [Crossref] [PubMed]
- Qiu T, Yu B, Xuan Y, et al. Vectorial localization of peripheral pulmonary lesion guided by electromagnetic navigation: a novel method for diagnostic surgical resection without dye marking. Thorac Cancer 2018;9:502-4. [Crossref] [PubMed]
- Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol 2019;14:445-58. [Crossref] [PubMed]
- Gu Y, Chen S, Shi J, et al. The introduction of electromagnetic navigation bronchoscopy for the diagnosis of small pulmonary peripheral lesions in an Asian population. J Thorac Dis 2017;9:2959-65. [Crossref] [PubMed]
- Kuo SW, Tseng YF, Dai KY, et al. Electromagnetic navigation bronchoscopy localization versus percutaneous CT-guided localization for lung resection via video-assisted thoracoscopic surgery: a propensity-matched study. J Clin Med 2019;8:E379. [Crossref] [PubMed]
- Chong Y, Cho HJ, Kang SK, et al. Outcomes of the tower crane technique with a 15-mm trocar in primary spontaneous pneumothorax. Korean J Thorac Cardiovasc Surg 2016;49:80-4. [Crossref] [PubMed]
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural history of pulmonary subsolid nodules: a prospective multicenter study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Nakashima S, Watanabe A, Obama T, et al. Need for preoperative computed tomography-guided localization in video-assisted thoracoscopic surgery pulmonary resections of metastatic pulmonary nodules. Ann Thorac Surg 2010;89:212-8. [Crossref] [PubMed]
- Awais O, Reidy MR, Mehta K, et al. Electromagnetic navigation bronchoscopy-guided dye marking for thoracoscopic resection of pulmonary nodules. Ann Thorac Surg 2016;102:223-9. [Crossref] [PubMed]
- Marino KA, Sullivan JL, Weksler B. Electromagnetic navigation bronchoscopy for identifying lung nodules for thoracoscopic resection. Ann Thorac Surg 2016;102:454-7. [Crossref] [PubMed]


