
Chronotropic incompetence could negatively influence post-operative risk assessment in patients before lung cancer surgery
Introduction
Chronotropic incompetence (CI) is broadly defined as the inability of the heart to increase its rate commensurate with increased activity or demand (1). The traditional equation used to predict maximal heart rate (HR) is 220 beats/minute – age (2,3). The definition of CI is not standardized among literature, though according to most definitions it is diagnosed when, during an incremental dynamic exercise test, HR fails to reach an arbitrary percentage (85%, 80% or less commonly 70%) of the age-predicted maximal HR (4-6).
Lung carcinoma is one of the tumours with the highest mortality and morbidity (7,8) and in patients with stage I, II and IIIA of non-small cell lung cancer surgical resection is the preferred method of treatment (9).
As a necessary part of the preoperative evaluation, patients are initially examined by pulmonary functional test. The standard part of the evaluation is the measurement of forced expiratory volume (FEV1) and lung diffusion capacity (TLCO) (10-12).
Patients with an FEV1 and TLCO >80% of the predicted values are able to undergo lung resection up to the extent of a pneumonectomy (10). Otherwise, if these values are not met, cardiopulmonary exercise testing (CPET) is indicated (13).
According to the most recent functional algorithm published by the American College of Chest Physicians (11) patients with a maximum oxygen consumption (VO2max) lower than 10 mL/kg/min or 35% of predicted, indicate a high risk for major anatomic resection. Conversely, VO2max values greater than 20 mL/kg/min or 75% of predicted, indicate low risk. The importance of the role of high technology exercise testing is supported by European guidelines (10) and a meta-analysis, which confirmed the importance and the ability of VO2max in predicting cardiopulmonary complications or mortality after pulmonary resection (14). Most of the studies agreed that a VO2max value below 10–15 mL/kg/min was to be regarded as a high-risk threshold for lung resection and that values above 20 mL/kg/min are safe for any kind of resection, including pneumonectomy (13).
However, the guidelines aforementioned do not mention the possibility that VO2max could be decreased by CI in patients. This could influence the result of the preoperative examination and the estimation of the postoperative risk of complications. The aim of this study was to evaluate the prevalence of CI in patients indicated for lung surgery and its possible effect on CPET parameters.
Methods
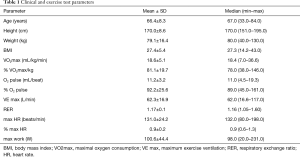
This study enrolled 154 consecutive patients (97 men) of average age 66.4±8.3 with newly diagnosed lung cancer indicated for lung surgery. In all patients, physical examination with the measurement of anthropometric parameters was performed.
All patients underwent CPET (cycle ergometry) using Vyntus CPET device. The CPET was performed 2 hours after a light meal, with patients having taken all their regular medication.
Exercise test: ramp test, maximum was defined as a plateau in oxygen consumption, RER >1.05.
All patients were assessed for the following parameters: max respiratory oxygen uptake (VO2max), oxygen pulse (O2 pulse), maximum exercise ventilation (VE max), respiratory exchange ratio (RER), maximum heart rate (max HR) and maximum work load (max work).
Predicted maximal HR was calculated using the traditional equation (220 – age). Three levels of CI were defined: 85% HRpred, 80% HRpred and 70% HRpred.
SPSS software version 15.0 (SPSS Inc., Chicago, USA) was used for statistical analysis. Spearman’s correlation analysis was carried out to evaluate the relationship between CI, beta-blocker status and other baseline variables. The normality of distribution was checked by the Shapiro-Wilk test. P<0.05 was considered statistically significant.
Statement of ethics approval
This study was approved by University Hospital and Faculty of Medicine and Dentistry, Palacky University Olomouc Ethics Committee as a number 39/19 and participants signed the Informed consent form.
Results
Baseline clinical characteristics together with exercise test parameters are shown in Table 1.
Full table
CI was present in following ratios: 85% HRpred—48.7%; 80% HRpred—39.6%; 70% HRpred—16.9%.
A significant negative correlation was found between VO2max, maximal HR and maximal work load among all CI groups (Table 2).

Full table
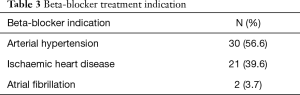
A total of 53 patients (34.4%) were being treated with beta-blockers. Indications for beta-blocker treatment are shown in Table 3.
Full table
The presence of CI significantly correlated with beta-blocker treatment and with age. To eliminate possible bias, a multivariate analysis was performed. After adjusting for age, a significant correlation was found between CI and beta-blocker treatment (P<0.0001).
Results of the Chi quadrate test and Mann-Whitney U test are presented in Tables 4-6.

Full table

Full table

Full table
Discussion
Exercise tolerance is an important determinant of quality of life and CPET is an important part of evaluation of patients before lung cancer surgery.
The increase in VO2 is achieved by a 2.2-fold increase in HR, a 0.3 fold increase in stroke volume and a 1.5 fold increase in arteriovenous oxygen difference (15). Therefore, it is obvious that CI is the most important cause of VO2 reduction. The clinical importance of CI outweighs the simple reduction of exercise tolerance and possible reduced quality of life. CI could also be the cause of worsened mortality and morbidity of patients.
The prevalence of CI in the general population is not known, with reported prevalence of CI in literature ranging between 9–89% (1). The reason for such a wide range in results is the obvious non-homogeneity of the definition of CI (differing equations and percentages of predicted HR).
Some data on CI in patients with chronic heart failure is available, where it is estimated to be between 25–70% (1) and in patients indicated for liver transplantation, CI is present in 50% (16).
Abbot et al. found that CI occurred in 29.9% of patients with a myocardial injury after noncardiac surgery (17).
Data concerning CI in patients before lung resection is missing and this is the first study to address this.
This study has found the prevalence of CI to be high at 48.7%; 39.6% and 16.9%; respectively according to the different definitions (85% HRpred; 80% HRpred; 70% HRpred), which is almost the same prevalence like in patients with chronic heart failure. If we consider that these patients are indicated for surgery, such a high level of CI seems to be clinically relevant.
In the past decades, several studies evaluated CI as a risk factor of different diseases. Hinkle et al. was the first to report the relationship between CI and increased cardiac and all-cause mortality (18) and patients with CI have an increased risk for developing atrial fibrillation (19).
CI is considered to be a negative prognostic risk factor in patients with chronic obstructive pulmonary disease (COPD) (20) though an improvement in CI was also found in COPD patients with severe emphysema after lung volume reduction surgery (21).
CPET is an essential part of the preoperative evaluation (10,11,13) of patients before lung cancer surgery and based on VO2 peak the overall risk is estimated.
The most important finding of this study is the decrease in the exercise tolerance of patients with CI in comparison with other subjects (and CI influence on VO2 peak is not reflected in current guidelines). This study found that not only maximal oxygen consumption but also maximal ventilation and work load were significantly reduced.
CI correlated with age. It is known that the resting HR is not variable throughout life, but there is an age-related decrease in maximum HR (1)—this effect was expected.
The presence of CI in this study strongly correlated with beta-blocker treatment, as was confirmed by previous studies (22), which could be an important consideration for the future evaluation of patients before lung cancer surgery. Also, in the group treated with beta-blocker a significant reduction in VO2 peak was found which supports the finding that beta-blocker induced CI reduces VO2 peak.
Indications for beta-blocker treatment were analysed and arterial hypertension was an indication for this treatment in almost 57% of patients. Thus, in patients with arterial hypertension beta-blocker use could be substituted safely with another antihypertensive in aim to reduce CI prevalence (23).
The next most common indication for beta-blocker use was ischemic heart disease. Generally, beta-blockers are considered to be an important part of the treatment of patients post myocardial infarction. However, data supporting its usage is derived from studies performed in the so-called ‘Pre-reperfusion era’. According to the review by Hong et al. (24) in the absence of a contemporary randomized control trial, this evidence imparts uncertainty regarding the current standard of care and suggests that it may be reasonable to discontinue use of beta-blockers in patients without impaired left ventricular function at 1-year post-myocardial infarction, who do not have another indication for their use. Therefore, in many cases could beta-blockers withdrawal or dose-reduction decrease CI level.
In this study, patients with CI were found to have significantly lower VO2max, which is an important part of the preoperative evaluation. Thus, it is possible that in a large group of patients’ CI is pharmacologically induced, leading to a reduction in overall exercise tolerance. In such patients, the estimated postoperative risk is calculated to be higher than the actual risk. In extreme cases, these patients will be disqualified from getting the lung resection and thus their prognosis will be worsened. Mainly in patients with arterial hypertension as an indication for beta-blockers, should a change in treatment and a repeat of the exercise examination be considered.
This study found that minute ventilation was significantly reduced in patients with CI. A previous study found that maximal ventilation is reduced in patients taking beta-blockers (25). It is not clear whether this is because of increased ventilatory efficiency, as the authors of the study aforementioned stated, or if it could have been simply a decremental effect.
This study has several limitations. In patients with CI and beta-blocker treatment a second subsequent CPET was not performed following the reduction or withdrawal of the medication so we are not able to prove that with the substitution or withdrawal of beta-blocker the level of CI will be reduced. On the other side, this effect is highly expected.
A new study is now under consideration to address the possible restoration of CI after beta-blocker withdrawal.
In the future, larger studies are needed to consider changes to the current guidelines in which CI will be addressed.
Conclusions
CI significantly decreases VO2max in patients before lung cancer surgery. It is strongly associated with beta-blocker treatment, which could negatively influence risk assessment. It is a matter for future discussion whether the evaluation of CI should be part of the preoperative care guidelines.
Acknowledgments
We would like to thank Dr. Zapletalova for statistical analysis.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by University Hospital and Faculty of Medicine and Dentistry, Palacky University Olomouc Ethics Committee as a number 39/19 and participants signed the Informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brubaker P, Kitzman D. Chronotropic incompetence: causes, consequences, and management. Circulation 2011;123:1010-20. [Crossref] [PubMed]
- Gulati M, Shaw LJ, Thisted RA, et al. Heart rate response to exercise stress testing in asymptomatic women: the st. James women take heart project. Circulation 2010;122:130-7. [Crossref] [PubMed]
- Astrand PO. Physical performance as a function of age. Jama 1968;205:729-33. [Crossref] [PubMed]
- Dresing TJ, Blackstone EH, Pashkow FJ, et al. Usefulness of impaired chronotropic response to exercise as a predictor of mortality, independent of the severity of coronary artery disease. Am J Cardiol 2000;86:602-9. [Crossref] [PubMed]
- Elhendy A, van Domburg RT, Bax JJ, et al. The functional significance of chronotropic incompetence during dobutamine stress test. Heart 1999;81:398-403. [Crossref] [PubMed]
- Lauer MS, Francis GS, Okin PM, et al. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999;281:524-9. [Crossref] [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016;5:288-300. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-e190S.
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65 Suppl 3:iii1-27. [Crossref] [PubMed]
- Brunelli A. Preoperative functional workup for patients with advanced lung cancer. J Thorac Dis 2016;8:S840-8. [Crossref] [PubMed]
- Benzo R, Kelley GA, Recchi L, et al. Complications of lung resection and exercise capacity: a meta-analysis. Respir Med 2007;101:1790-7. [Crossref] [PubMed]
- Higginbotham MB, Morris KG, Williams RS, et al. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res 1986;58:281-91. [Crossref] [PubMed]
- Singhal A, Mukerji AN, Thomaides A, et al. Chronotropic incompetence on dobutamine stress echocardiography in candidates for a liver transplant. Exp Clin Transplant 2013;11:546-53. [Crossref] [PubMed]
- Abbott TEF, Pearse RM, Beattie WS, et al. Chronotropic incompetence and myocardial injury after noncardiac surgery: planned secondary analysis of a prospective observational international cohort study. Br J Anaesth 2019;123:17-26. [Crossref] [PubMed]
- Hinkle LE Jr, Carver ST, Plakun A. Slow heart rates and increased risk of cardiac death in middle-aged men. Arch Intern Med 1972;129:732-48. [Crossref] [PubMed]
- O'Neal WT, Qureshi WT, Blaha MJ, et al. Chronotropic Incompetence and Risk of Atrial Fibrillation: The Henry Ford ExercIse Testing (FIT) Project. JACC Clin Electrophysiol 2016;2:645-52. [PubMed]
- González-Costello J, Armstrong HF, Jorde UP, et al. Chronotropic incompetence predicts mortality in severe obstructive pulmonary disease. Respir Physiol Neurobiol 2013;188:113-8. [Crossref] [PubMed]
- Armstrong HF, Gonzalez-Costello J, Jorde UP, et al. The effect of lung volume reduction surgery on chronotropic incompetence. Respir Med 2012;106:1389-95. [Crossref] [PubMed]
- Witte KK, Cleland JG, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart 2006;92:481-6. [Crossref] [PubMed]
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018;36:1953-2041. [Crossref] [PubMed]
- Hong J, Barry AR. Long-Term Beta-Blocker Therapy after Myocardial Infarction in the Reperfusion Era: A Systematic Review. Pharmacotherapy 2018;38:546-54. [Crossref] [PubMed]
- Wolk R, Johnson BD, Somers VK, et al. Effects of beta-blocker therapy on ventilatory responses to exercise in patients with heart failure. J Card Fail 2005;11:333-9. [Crossref] [PubMed]


