Impact of fractional exhaled nitric oxide on the outcomes of lung resection surgery: a prospective study
Introduction
Lung resection is indicated for several conditions, including curative resection for lung cancer and limited resection for diagnostic purposes. It has become less invasive owing to advancement in surgical procedures. However, patients with respiratory comorbidities are susceptible to loss of function after surgery and a high proportion of postoperative complications. Therefore, risk assessment and perioperative management are essential, depending on the disease.
Some studies in patients with interstitial pneumonia have reported the efficacy of perioperative pirfenidone. They also reported that sialylated carbohydrate antigen (KL-6) levels and the diffusing capacity of carbon monoxide are poor prognostic factors (1-3). However, few conventional indicators are available for chronic obstructive pulmonary disease (COPD), which has a higher prevalence, and further universal risk factors should be investigated.
The novel concept of asthma-COPD overlap has been proposed recently, which presents with a combination of the features of chronic lung disease. Airway inflammation has been studied with respect to its contribution to disease progression (4). Airway inflammation is quantified using fractional exhaled nitric oxide (FeNO), which is widely used in daily practice, because it can be easily measured (4), but its impact on lung resection remains unknown.
We hypothesized that a state of high airway inflammation is associated with adverse effects on the clinical course. The purpose of this prospective study was to identify the potential risk in patients using FeNO and examine the clinical significance of airway inflammation measurement in patients who underwent lung resection.
Methods
Patients and study design
This single-center, prospective study was conducted at Shiga University of Medical Science Hospital and was approved by the appropriate institutional review boards (protocol number 29-175). Written informed consent was obtained from each participant.
This study included adult patients younger than 90 years of age, who underwent elective lung resection at the Department of General Thoracic Surgery, Shiga Medical University of Medical Science (Shiga, Japan) between September 2017 and March 2019. We measured FeNO in participants who provided informed consent, in addition to routine preoperative blood, physiological function, and imaging tests. The exclusion criteria were as follows: patients aged under 20 years, those with a history of treatment for infection within the past 1 month, those suspected of active pulmonary infection on admission, those using immunosuppressants, and patients in whom FeNO examination could not be performed satisfactorily.
Study setting
The participants’ airway inflammation and subjective symptoms were evaluated once before and on days 1, 3, 5–7 after surgery, for a total of 4 times. Airway inflammation was measured as FeNO using NIOX VERO® (NOV, Aerocrine, Solna, Sweden). Measurements were made during the day, except for 2 hours after meals and after waking up. We simultaneously interviewed patients regarding their symptoms, which were recorded with the COPD assessment test (CAT) (5) using only 3 items (cough, sputum, dyspnea) from the CAT questionnaire, considering the patients’ inconvenience after surgery. The total score of the modified CAT was 21.
The primary endpoint was the relationship between postoperative morbidity in the hospital and preoperative FeNO. The Clavien-Dindo and postoperative pulmonary complications (PPCs) classifications were used to evaluate morbidity (6,7). Major complications were defined as grade 3 or higher on the Clavien-Dindo classification. One of the secondary endpoints was to clarify the perioperative dynamics of airway inflammation. Postoperative FeNO levels were described with respect, to preoperative levels, and the difference was analyzed. Another secondary endpoint included the investigation of the relationship between increased airway inflammation and respiratory events comprising additional medical treatment within 30 days and readmission within 90 days of surgery.
We also prospectively recorded the following variables: sex, age, body mass index, smoking history (Brinkman index), medical history [Charlson Comorbidity index (CCI) score] (8), tumor diameter measured using computed tomography, blood test findings (carcinoembryonic antigen, KL-6, brain natriuretic peptide, and hemoglobin A1c), respiratory function [vital capacity (VC), forced expiratory volume in 1 second (FEV1.0) and their predicted values (%VC, %FEV1.0)], surgery-related findings (procedure, approach, duration time, and blood loss), and pathological findings of lung tumor.
Statistical analysis
Statistical analysis was performed using SPSS software, version 22.0 (IBM, Inc., CA, USA). All continuous values were expressed as mean ± standard deviation (SD). The peak value of postoperative FeNO was used as the representative value, when it was used as a variable during statistical analysis. A multivariate logistic regression model was used to analyze the predictors of postoperative complications and additional respiratory-event related treatment. Baseline variables with P values <0.250 obtained from univariate analysis were included in the multivariable models. The chi-squared test was used for comparing categorical variable. The t-test and Mann-Whitney’s U test were used for comparing continuous variables. Pearson’s correlation coefficient and linear regression analysis were used to determine the relationship between postoperative FeNO levels and the modified CAT score. P values <0.05 were considered to be statistically significant.
Results
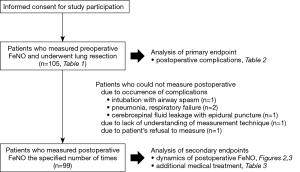
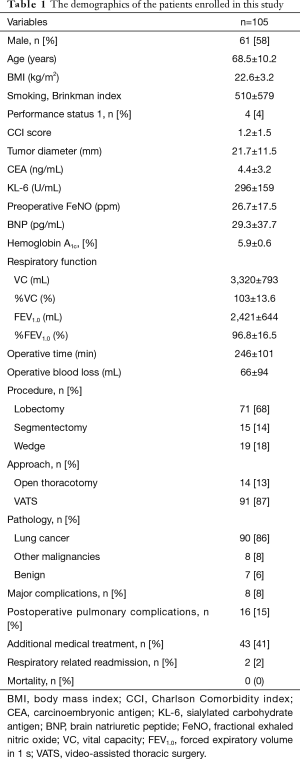
Figure 1 summarizes the patients who participated in this study (n=105). The characteristics of all patients are presented in Table 1. Men were predominant in the study population. The proportion of primary lung cancer was the highest. The percentage of the anatomical lung resection approach was 87%. None of the patients required extended surgery owing to intraoperative findings. Lobectomy was performed as radical surgery for lung cancer, and segmentectomy and wedge resection were adopted as conservative approaches for diagnosis or treatment.
Full table
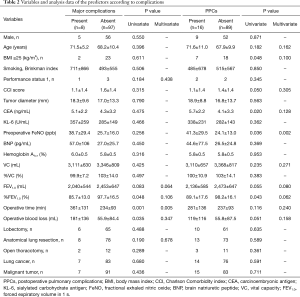
Major complications developed in 8 patients (8%), which included chylothorax (n=2), pulmonary fistula (n=2), hypoxia (n=3), and bronchospasm (n=1). PPCs developed in 16 patients (15%). PPCs consisted of pneumonitis and bronchiolitis (n=6), bronchospasm (n=3), hypoxia (n=3), pulmonary fistula (n=2), and atelectasis (n=2). Additional medical treatment within 30 days of surgery comprised cough medication alone (n=26), antibacterial agents (n=8), antiviral drugs (n=1), intravenous corticosteroids (n=3), and inhalational corticosteroids (n=3). Two patients were readmitted within 90 days of surgery, owing to bacterial pneumonia and acute exacerbation of interstitial pneumonia, respectively. The 90-day mortality was 0. The following predictors of postoperative morbidity were identified among all patients, based on statistical analysis (Table 2). Multivariate analysis revealed that operating time was a significant predictor of major complications [P=0.004, odds ratio (OR): 1.012, 95% confidence interval (CI): 1.004–1.021] and preoperative FeNO was a significant predictor of PPCs (P=0.002, OR: 1.004, 95% CI: 1.016–1.074) (Table 2).
Full table
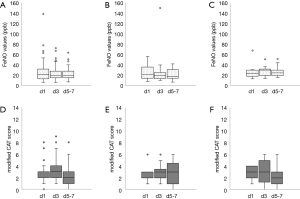
The distribution of perioperative FeNO levels is shown in Figure 2. The mean FeNO level increased significantly after surgery (P=0.011) in all patients, but no significant difference was observed after dividing it by surgical procedure. Figure 3 shows the changes in postoperative FeNO levels and modified CAT scores. FeNO levels peaked on the first day postoperatively in the anatomic lung resection group and on the third day postoperatively in the wedge resection group. The results of the correlation analysis between postoperative FeNO and modified CAT scores were P=0.250, r=0.250, P=0.625, r=−0.556 and P=0.819, r=0.280 in the lobectomy, segmentectomy and wedge resection groups, respectively.

Statistical analysis was performed to determine the relationship between the secondary endpoints and variables, including postoperative FeNO (Table 3). Multivariate analysis revealed that postoperative FeNO was a significant predictor of additional medical treatment within 30 days of surgery (P=0.001, OR: 1.068, 95% CI: 1.028–1.110). No predictors for readmission were discovered on univariate and multivariate analyses.
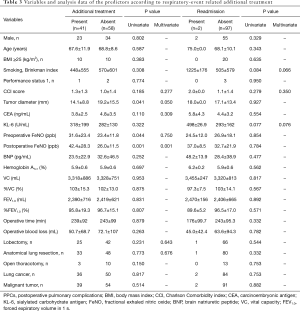
Full table
Discussion
The principal finding of this study was that high preoperative FeNO affected the development of PPCs after lung resection surgery. We also demonstrated postoperative FeNO dynamics and that patients with elevated levels were more likely to require additional medical treatment.
FeNO, which is significantly correlated with sputum eosinophils (9), can reflect Th2-driven airway inflammation, which results in the upregulation of inducible nitric oxide synthase in the bronchial epithelium (10,11). FeNO is regarded as an indicator of respiratory disease with high bronchial inflammation, including asthma and ACO (12). It is widely used since it can be measured easily (13). Clinical research using conventional methods is generally avoided because forced cough and spirometry in the postoperative acute phase can lead to worsening of the patient’s condition. However, our evaluation method overcame these disadvantages and may be used in clinical settings.
We studied multiple surgical procedures and found that prolonged procedures such as lobectomy for lung cancer may have been responsible for major complications (Table 2). The Clavien-Dindo classification covers a wide range of surgical complications. However, it cannot adequately represent respiratory status in detail (14-16). We used PPCs in order to clearly define these individual adverse effects, which helped to prove our hypothesis. PPCs are especially associated with early postoperative mortality (17), and measurement of preoperative FeNO was valuable in preventing respiratory complications and subsequent deterioration.
We analyzed perioperative FeNO dynamics but failed to observe any statistical difference between each surgical procedure. Initially, we thought that the postoperative increase in FeNO values would be prominent for generally invasive procedures, including lobectomy. However, the increase was most pronounced (P=0.068) in the wedge-resection group, and unlike the other groups, it peaked on the third day, postoperatively. Complications and steroid therapy after surgery may have been responsible for false-negative airway inflammation levels in some patients. We assumed that neutrophilic inflammation caused by bronchial incision progressed soon after surgery and eosinophilic inflammation was suppressed in the anatomical resection group. Pain caused by surgical factors such as wound size and approach was thought to have contributed to the results of the symptom scores.
The results presented in Table 3 suggest that the local exacerbation of eosinophilic inflammation after surgery increased the need for additional treatment. Aggressive anti-inflammatory treatment, including early steroid inhalation therapy, may regulate the environment of the lower airway and improve the patient’s condition quickly. Further investigations with a larger sample size are required, to clinically implement perioperative management based on FeNO.
This was the first prospective study to evaluate and analyze perioperative airway epithelial inflammation and surgical outcomes in patients who underwent lung resection in a quantitative manner. The basic objective of this study was to explore patients at an increased risk and to investigate the feasibility of perioperative management strategies using FeNO. This was a single-center clinical study. Thus, there may have been selection, measurement, and follow-up bias. Moreover, cases involving severe invasive surgery, such as bronchoplasty and pneumonectomy were not included in this study; therefore, other risk factors may require complete consideration in patients requiring extended surgery.
In summary, our analysis demonstrated that perioperative FeNO was a significant predictor of surgical outcome among patients who underwent lung resection. Measurement of FeNO is a simple and useful method for preventing subsequent deterioration status in such patients.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This single-center, prospective study was conducted at Shiga University of Medical Science Hospital and was approved by the institutional review board (protocol number 29-175). Written informed consent was obtained from each participant.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sato T, Teramukai S, Kondo H, et al. Impact of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-1611.e3. [Crossref] [PubMed]
- Berry MF, Jeffrey Yang CF, Hartwig MG, et al. Impact of pulmonary function measurements on long-term survival after lobectomy for stage I non-small cell lung cancer. Ann Thorac Surg 2015;100:271-6. [Crossref] [PubMed]
- Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study). Respir Res. 2016;17:90. [Crossref] [PubMed]
- Global initiative for Asthma, Global Initiative for Chronic Obstructive Lung Disease. Diagnosis of Diseases of Chronic Airflow Limitation: Asthma, COPD, and Asthma- COPD Overlap Syndrome (ACOS) [Accessed Sep 30, 2019]. Available online: http://goldcopd.org/asthma-copd-asthma-copd-overlap-syndrome/
- Casanova C, Marin JM, Martinez-Gonzalez C, et al. Differential effect of modified medical research council dyspnea, COPD assessment test, and clinical COPD questionnaire for symptoms evaluation within the new GOLD staging and mortality in COPD. Chest 2015;148:159-68. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a surgery. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth 2017;118:317-34. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Tajiri T, Niimi A, Matsumoto H, et al. Prevalence and clinical relevance of allergic rhinitis in patients with classic asthma and cough variant asthma. Respiration 2014;87:211-8. [Crossref] [PubMed]
- Iijima H, Duguet A, Eum SY, et al. Nitric oxide and protein nitration are eosinophil dependent in allergen-challenged mice. Am J Respir Crit Care Med 2001;163:1233-40. [Crossref] [PubMed]
- Asano T, Takemura M, Fukumitsu K, et al. Diagnostic utility of fractional exhaled nitric oxide in prolonged and chronic cough according to atopic status. Allergol Int 2017;66:344-50. [Crossref] [PubMed]
- Niimi A, Matsumoto H, Mishima M. Eosinophilic airway disorders associated with chronic cough. Pulm Pharmacol Ther 2009;22:114-20. [Crossref] [PubMed]
- Kharitonov SA, Gonio F, Kelly C, et al. Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J 2003;21:433-8. [Crossref] [PubMed]
- Shander A, Fleisher LA, Barie PS, et al. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med 2011;39:2163-72. [Crossref] [PubMed]
- Canet J, Gallart L, Gomar C, et al. ARISCAT Group. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010;113:1338-50. [Crossref] [PubMed]
- Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg 2017;152:157-66. [Crossref] [PubMed]
- Vetter TR, Ivankova NV, Goeddel LA, et al. UAB Perioperative Surgical Home Group. An analysis of methodologies that can be used to validate if a perioperative surgical home improves the patient-centeredness, evidence-based practice, quality, safety, and value of patient care. Anesthesiology 2013;119:1261-74. [Crossref] [PubMed]