
ATTACHED, DETACHED and WITHOUT inhaler technique coaching tools to optimize pMDI use competence, asthma control and quality-of-life in asthmatic adults
Introduction
Asthma, an obstructive lung condition, is a burden on both healthcare systems and societies worldwide (1). The prevalence of asthma has increased by 4% in adults over a 20 year period (2). The patients’ poor- or non-adherence to their inhaled therapeutic regimens can lead to catastrophic clinical and socioeconomic consequences (3,4). It has been reported that non-adherence in chronic obstructive pulmonary disease (COPD) and asthma patients is high (3,5). The root cause is complex and multifactorial in nature that can be broadly associated with patient, society and treatment aspects (4). This latter aspect covers the methods of administration (including multiple inhaler prescriptions, correct inhaler technique and training), dosing regimens and adverse effects (4).
A correct pressurized metered dose inhaler (pMDI) technique is critical to deliver the inhaled dose to its pharmacologic site in the lung periphery (6,7). For adequate lung deposition, it is crucial for patients to coordinate the start of a slow and deep inhalation flow with the pMDI canister actuation (known as hand-lung coordination) and to follow that with a few seconds of breath holding (8-10). However, the majority of patients perform these two critical pMDI manoeuvres poorly (8,11-13) with fast peak inhalation flow (PIF) through the inhaler (13,14). This would, accordingly, maximize the oropharyngeal and central lung depositions increasing the risks of either over- or unnecessary use of inhaled medicines including corticosteroids (15).
Owing to differences in study design, 12–93% of the patients have been reported to make critical pMDI technique errors, and 45.7–100% had overall pMDI use issues (16). Poor inhaler technique among patients is prevalent and, despite various traditional training approaches, has not improved over the past four decades (11). Educational interventions on correct inhaler use—including verbal inhaler training (VT)—are effective for the short term only (17). In real-life, the VT which patients might receive during their routine clinic visits deteriorates gradually with time at rates related to the patients’ abilities to digest and recall the VT, treatment non-adherence and the frequency of the VT sessions (8). Additionally, poor healthcare professionals’ (HCPs) knowledge and competency in correct inhaler use may impair their ability to adequately assess and train their patients in the use of their inhaled products (18). Inhaler technique training devices designed with a feedback mechanism that informs of a correctly performed inhalation manoeuvre can be a step forward in helping both HCPs and patients optimize and monitor their inhaler handling (10,13,14,19).
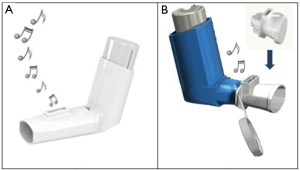
Trainhaler® (THR), Clement Clarke International Limited, UK (CCI), is a recent pMDI training tool (Figure 1A). When a patient is trained with the THR, it produces two audible feedback sounds; a “whoosh” noise mimicking that of a real puff released from an actuated pMDI and a whistle sound when the correct, slow inhalation flow through the THR is achieved. Patients are then instructed to simulate the THR training when using their real therapeutic pMDI. Additionally, CCI has also developed the Flo-Tone® CR (FTCR) as both a mini-spacer and pMDI training tool (Figure 1B). Once attached to the mouthpiece of the inhaler, the FTCR produces a whistle sound once the patient starts a slow inhalation through the pMDI giving the feedback signal to actuate the puff. The patient is trained to keep the whistle sound going throughout a slow and deep inhalation via their “pMDI plus FTCR” setup. The present work aimed to compare the traditional pMDI VT with the novel THR and FTCR tools in adult patients with asthma. The short- (immediate) and long-term reflections of the three inhaler training approaches on the pMDI technique and inhalation were primarily evaluated alongside various secondary clinical and health outcomes.
Methods
A prospective, investigational, parallel-group, two clinic-visit, randomized study was conducted to assess the conventional verbal pMDI technique training (VT) approach against the two recently introduced pMDI training tools; the THR and FTCR, in outpatient adults with asthma. The relatively short- and long-term impact on the participants’ overall 11-step pMDI technique (Figure 2) with emphasis on the critical hand-lung coordination manoeuvre including a slow and deep inhalation profile through the pressurized inhaler were the primary study outcomes. Whilst, changes in the patients’ lung function, asthma control and quality-of-life were the secondary clinical measures. The study involved two clinic-based visits for each recruited patient, with a 6-week gap (+2 weeks window) between these visits.
Eighteen to 60 year-old patients with stable asthma, who were originally prescribed and using pMDI therapy (without a spacer device) including a corticosteroid inhaler for at least three months prior to enrolment, and had poor pMDI use (defined as a poor hand-lung co-ordination with a PIF >60 L/min) were eligible to participate in this research. Subjects were excluded if they had an acute asthma exacerbation or had received oral corticosteroid treatment one month prior to recruitment, had other health conditions adversely affecting their respiratory system or affecting their ability to use their pMDI, the THR or the FTCR tools by themselves. Patients were also excluded if they had any hearing issues making them unable to recognize the audible feedback of the THR and FTCR. Screened patients with adequate pMDI use including PIFs ≤60 L/min through their inhalers were excluded from participation. Eligible patients that agreed to take part signed an informed consent for this research.
On visit 1, the age, gender, height, lung function [forced expiratory volume in 1 second (FEV1)] and asthma medications of each participant were taken. Each subject was then asked to complete both Juniper’s Asthma Control Questionnaire (ACQ) (20,21) and Mini Asthma Quality of Life Questionnaire (mini-AQLQ) (22). The participant was then asked to demonstrate their usual pMDI technique using a placebo inhaler (baseline inhaler use) which was checked against the 11-step technique. Afterwards, the PIF (L/min) through pMDI was measured using the In-Check® flow meter (CCI). The participants were sequentially randomized into the VT, THR or FTCR groups according to a pre-study randomization list that was created online in which the three inhaler training approaches were distributed randomly in balanced blocks of six (Sealed Envelope Ltd. Create a blocked randomisation list. Available from: https://www.sealedenvelope.com/simple-randomiser/v1/lists). The VT subjects were verbally trained on the correct pMDI steps described in Figure 2. The THR and FTCR subjects received the pMDI technique training (Figure 2) by practicing their assigned inhaler training device according to the manufacturer’s leaflet instructions. The VT, THR and FTCR training at visit 1 continued as described above until all subjects adequately demonstrated the correct pMDI technique including a PIF ≤60 L/min. To evaluate the short-term inhaler training effect, pMDI hand-lung coordination and PIF were assessed about 1 hour later before the subjects were discharged until the second visit. The THR patients were given their THRs to take home to practice with 2–3 times a day just before taking their pMDI therapy. Whilst, in FTCR group the tools were attached to the patients’ therapeutic pMDIs to take home and use while connected with their inhalers.
On visit 2, each participant demonstrated their own pMDI technique using a placebo inhaler (checked against the 11-step technique) and had their PIF through the pMDI measured. FEV1 was evaluated. The ACQ and mini-AQLQ were also completed. The participant’s asthma medications over the study follow-up period were checked for and the reason for changes, if any, was obtained before the subjects were discharged from the study. To minimize inter-individual variability, one well-trained, experienced researcher (R.J.A.) did take/measure all the study outcomes for all the participants on the two study occasions.
The research protocol was initially approved by the Research Ethics Committees at the Jordan University Hospital (Ref: 10/2015/1523) and at the Ministry of Health (Ref: MOH-REC-150131), Amman, Jordan. The study was conducted according to the Helsinki Declaration and Good Clinical Practice (ICH/GCP) Guidance and its updates.
Statistical analyses
The statistical analysis was carried out using the Statistical Package for Social Sciences software (IBM SPSS for windows, Version 20). Descriptive statistics were presented as mean (standard deviation), median (25%; 75% quartiles), and frequencies and percentages, as appropriate. Parametric and normal distribution behaviour of the study data were firstly checked for using histograms and Kolmogorov-Smirnov and Shapiro-Wilk tests before choosing the appropriate comparative statistical test. Comparisons within the same study group were performed using the related (paired)-samples t-test (for parametric data) and the Wilcoxon test (for non-parametric data). Comparisons between the VT, THR and FTCR groups (at either visit 1 or visit 2) were performed using the independent-sample t-test (for parametric data) and the Mann-Whitney U test (for non-parametric data). A P value <0.05 was considered statistically significant for any difference.
Results
Two hundred and twenty-four stable asthmatic adults were screened in the outpatient clinics involved with this study. Ninety-two eligible subjects agreed to sign informed consent forms and completed visit 1 procedures. The participants were randomized into the VT (n=33), THR (n=24) and FTCR (n=35) pMDI training groups. The patients were screened and randomized into the study sequentially. Table 1 summarises their demographics, lung function (FEV1% predicted) and asthma severity. Pairwise comparisons showed no significant differences (P>0.05) in the participants’ age and height between the study groups (Mann-Whitney U test), however the FTCR patients were significantly (P<0.05) older than THR patients; mean 44.7 and 36.0 years, respectively. The number of female participants was higher than that of the male ones. Forty-six patients attended the second study visit; VT (n=15), THR (n=13) and FTCR (n=18). No changes in the participants’ asthma medications were noticed throughout the study.

Full table
Table 2 presents the short-term inhaler training impact on the PIF (L/min) through the pMDI during visit 1. The between-group PIF comparisons (the Mann-Whitney test) showed that the VT, THR and FTCR groups had statistically comparable (P>0.05) baseline (pre-training) PIF via pMDI. One hour post-training, the VT group had similar (P>0.05) PIF to that of both the THR and FTCR groups. Whilst, the FTCR subjects had statistically lower PIF through pMDI than the THR subjects (Mann-Whitney U =283.0; z-statistic =−2.1, P=0.032). Additionally, Table 2 presents the within-group changes (Δ) in PIF pre- and 1 hour post-inhaler training alongside their Wilcoxon test statistical significance.

Full table
For the long-term impact of the inhaler training methods on the PIF through the pMDI, the Wilcoxon test showed that the decrease in mean PIF between visit 1 (165.0±80.2) and visit 2 (115.3±77.5) within the VT group (n=15) was statistically significant: z-statistic =−3.3 (P=0.001). Within the THR (n=13), the decrease in mean PIF between visit 1 (190.0±74.4) and visit 2 (94.6±32.0) was statistically significant: z-statistic =−3.0 (P=0.003). For the FTCR (n=18), the decrease in mean PIF between visit 1 (159.4±84.3) and visit 2 (96.1±48.0) was also statistically significant: z-statistic =−3.3 (P=0.001). The pairwise between-group Mann-Whitney U test comparisons of the PIF for the participants that completed the two visits have shown non-significant differences (P>0.05) at visit 1, visit 2 and for the ΔPIF among all groups. Figure 3 shows the improvement in individual PIF through pMDI at visit 1 (pre- and post-pMDI training) and visit 2 for the patients in 3 study groups.

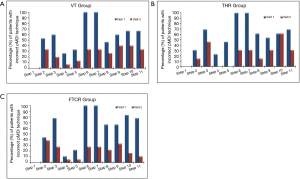
The subjects’ 11-step pMDI technique was evaluated at both visit 1 (baseline) and visit 2. The median (quartiles) of the incorrect pMDI technique steps at visit 1 was: 7 (4; 7) for the VT, 6 (3.5; 8.5) for the THR and 7 (5; 8) for the FTCR. Whereas, at visit 2 the median (quartiles) of the incorrect pMDI technique steps was: 2 (0; 4) for the VT, 2 (1; 4) for the THR and 1.5 (1; 3) for the FTCR. The percentages of the participants with incorrect pMDI steps at both visits, for the VT, THR and FTCR groups are presented in Figure 4A,B,C, respectively. Statistically, the Wilcoxon test showed significant improvements in the pMDI technique within the VT (z-statistic =−2.77; P=0.006), within the THR (z-statistic =−3.07; P=0.002) and within the FTCR (z-statistic =−3.56; P<0.001) groups. The Mann-Whitney U test showed that the patients in the three study groups had similarly (P>0.05) poor pMDI technique at enrolment, and that the VT, THR and FTCR pMDI training approaches comparably improved inhaler use by visit 2 (P>0.05).
Pairwise independent samples t-test comparisons of the FEV1% predicted showed no significant differences (P>0.05) among all study groups at either visit 1 or visit 2. For the subjects that completed the two study visits, the related-samples t-test showed no significant changes (P>0.05) in the FEV1% predicted between the two visits within each study group.
Within the VT group, the related-samples t-test showed that the improvement in the ACQ scores between visit 1 (M =1.78; SD =1.07) and visit 2 (M =1.58; SD =1.44) was statistically non-significant (P=0.408). For the THR group, the ACQ difference between visit 1 (M =2.54; SD =1.29) and visit 2 (M =2.14; SD =1.14) was also statistically non-significant (P=0.146). Similarly, the decrease in the ACQ scores within the FTCR group between visit 1 (M =1.93; SD =1.41) and visit 2 (M =1.82; SD =1.00) was statistically non-significant (P=0.766). Table 3 presents the frequencies of the VT, THR and FTCR patients over the asthma control cut-point categories of the ACQ (21). The between-group, independent-samples t-test showed no significant differences (P>0.05) in asthma control among the VT, THR and FTCR at either visit 1 or at visit 2.

Full table
The participants completed the 15-question mini-AQLQ covering four asthma-related quality of life domains. Table 4 presents the mean (SD) of the mini-AQLQ scores at visits 1 and 2, as well as the within-group comparison of mini-AQLQ scores. Additionally, the pairwise independent-samples t-test of the mini-AQLQ scores at visit 1 and at visit 2 revealed nonsignificant differences (P>0.05) among the VT, THR and FTCR.
Full table
Discussion
In asthma management, matching the right inhaler device to the right patient is vital to maximize therapeutic outcome (1,4). The proper choice of an inhaler should take into consideration both the device design and, equally important, the patients’ characteristics (4,23-25). Unfortunately, HCPs commonly overlook their patients’ initial willingness and ability to correctly use the prescribed inhaled products (10,18). Long-term improper inhaler technique is associated with poor inhaled medicine adherence which eventually increases the risks of emergency department visits (62%), hospitalizations (47%) and subsequently additional use of antimicrobial (50%) and oral corticosteroid (54%) therapies (4). Although valved holding chambers (or spacer devices) can complement patients’ poor pMDI technique and improve lung deposition, many patients find them inconvenient to use with their pressurized inhalers (6,7). In the healthcare setting, verbal inhaler counselling is the conventional approach to patient education. The current study aimed to evaluate and compare three pMDI technique training approaches in adults with stable asthma. These were the VT, THR and FTCR tools. The primary outcome measure was the relatively short- and long-term overall pMDI technique (including PIF through the inhaler). The impact of the study interventions on lung function, asthma control and quality-of-life were secondary clinical outcomes.
All three study groups had similar baseline (pre-training) overall pMDI technique errors; where 61.3% (VT), 61.5% (THR) and 65.0% (FTCR) patients made ≥2 errors. A systematic review (cross-sectional 19 studies) has reported a range of overall pMDI error frequency between 45.7% and 100% (16). After 6–8 weeks (visit 2), the three pMDI training interventions did significantly, and equally, improve the patients’ inhaler use; where only 28.0% (VT), 26.2% (THR) and 21.7% (FTCR) patients continued to make at least 2 pMDI errors. However, the hand-lung coordination that is accompanied with a slow and deep inhalation profile (Steps 6 and 7) are considered the critical pMDI manoeuvres that would significantly affect the aerosol lung deposition (26,27) and thus asthma control (28). All of our subjects (100%) performed these two steps incorrectly at recruitment. The frequency of the critical pMDI errors had been previously reported 12–93% (16). In the present work, only 33.3% (VT), 30.8% (THR) and 27.8% (FTCR) continued to poorly synchronize the start of slow inhalation with canister actuation by the end of the study (visit 2).
A slow PIF value through the pMDI is still controversial. It is generally agreed that 30 L/min is the ideal PIF and that an inhalation range between 30 and 60 L/min is the target that patients should be educated/trained to achieve (29,30). However, PIFs ≤90 L/min were found to be realistically slow enough for a therapeutically adequate lung deposition (31,32), whilst PIFs >90 L/min are considered inappropriately fast. However, in real-life the majority of the pMDI users had PIFs that were much higher than 100 L/min (9,12,13,33). Our findings were no exception, the pre-training mean PIFs were 175.2, 187.1 and 158.9 L/min for the VT, THR and FTCR patients, respectively (P>0.05). The current pMDI training interventions did efficiently (P<0.001) improve mean PIF when re-assessed about 1 h post-training; 81.8, 87.1 and 72.6 L/min, respectively. Moreover, the participants did maintain their trained, slow PIFs up to 8 weeks post-training; 115.3 (VT), 94.6 (THR) and 96.1 (FTCR) L/min. Previously, it had been shown that 20–50% of the verbally counselled patients reverted back to their old habits of poor pMDI technique within as short as 1 to 30 days after their inhaler training sessions (34,35). Our VT patients showed a similar trend as they seemed to forget the VT they received on visit 1, and thus their overall pMDI steps and PIF began to gradually, yet insignificantly, deteriorate throughout the follow-up period compared with the THR and FTCR patients. Another critical pMDI error that can reduce the peripheral lung deposition is the failure to immediately hold the breath for a few seconds after the aerosol inhalation (29,36). At recruitment, 60% (VT), 54% (THR) and 67% (FTCR) patients failed to demonstrate adequate breath-holding post inhalation. The three training approaches, though, significantly improved this manoeuvre; 40%, 31% and 33%, respectively, which is expected to be positively reflected on lung dose.
Clinically, all the participants had comparable lung function (FEV1% predicted) at recruitment. Despite the significant improvement in the pMDI use and inhalation flow in all inhaler training groups, the FEV1% predicted remained the same within and across the VT, THR and FTCR patients. The mild to moderate [mean (SD) FEV1% predicted: 76.3 (20.3)] stable asthma of the participants coupled with the relatively short follow-up period might have possibly limited any opportunity to notice significant reflections on lung function. In line with our findings, previous studies have reported that the FEV1 was not affected by the post-training pMDI technique improvement in asthmatic children (12,13) and adults (9,13).
In the real world, asthma control deterioration can be associated with pressurized inhaler mishandling (28,37), particularly with poor pMDI coordination (28). Of all our recruits, only 14 (15.2%) patients self-judged their asthma as being well-controlled (ACQ score ≤0.75). However, despite that each of the inhaler training methods had a significant positive impact on the pMDI technique and PIF, the patients’ reported asthma control scores (ACQ) remained similar (P>0.05) to those at study entry. Although improved in all groups, the changes in the ACQ scores were below the clinically minimal important difference (MID) of 0.5. The study sample that already had a stable asthma, the 6–8 weeks follow-up period and the “during the past 1-week” timeframe that the ACQ gives to the patients to recall their asthma-related experiences to score their responses might explain the virtually plateaued asthma control. Previously, no change was observed in asthma control, assessed by the three key questions of the British Royal College of Physicians (RCP) or the ACQ, that should have been anticipated by the improved use of the pMDI either alone (13) or with a spacer (38).
Asthma control tools correlate well with and are predictive of the patients’ asthma-related quality of life questionnaire outcomes (20,39). In agreement with the current ACQ outcomes, the overall mini-AQLQ and its four quality of life domains remained unchanged (P>0.05) throughout the study within the VT, THR and FTCR groups, with score changes below the MID threshold to be considered clinically important. A previous inhaler technique interventional study in homogeneous, stable asthmatics has shown quality of life behaviours similar to the current work (13). However, patients recruited with more severe and variable asthma stability did have a self-perception of an improved quality of life which might have been augmented by the longer term (up to 12 months) repeated VT reinforcement (9,40).
The current research was limited by the small number of participants particularly those who attended the second study visit. In this regard, every effort was made to have all the volunteers return to the study clinics by contacting them via both phone calls and text messages. Work commitments, travelling abroad, residence reallocation or personal reasons were, however, behind their absences. Moreover, this academically conducted study received a very small fund which restricted the number of recruiting clinics, participants and the study follow-up period. Longer observation might have shown more significant positive reflections related to using the THR and FTCR pMDI trainers compared with the conventional VT.
Conclusions
Consistent, good inhaler technique is vital to deliver therapeutically adequate lung doses of inhaled products. Currently, our investigated VT, THR and FTCR pMDI training approaches did significantly improve the asthmatic patients’ overall inhaler use including the vital coordinated slow inhalation maneuver, and maintained their asthma control and quality-of-life. Regular inhaler technique monitoring is scarce particularly in busy healthcare settings, thus the patients’ received VT fades usually way over time. The novel THR and FTCR pMDI training tools are two available options to complement and enhance the VT. Equally important, the THR and FTCR devices are retained by the patients as their self-monitoring, all-time personal trainers that can boost and maintain their correct pMDI technique between their routine clinic visits.
Acknowledgments
The authors are immensely thankful to all the patients who participated in this study. Thank you to Clement Clarke International Limited, United Kingdom, for unconditionally providing the Trainhaler and Flo-Tone CR devices free-of-charge. This academically-initiated, designed and conducted research study was unconditionally, partially supported by Clement Clarke International Limited, UK (CCI). CCI provided the Trainhaler and Flo-Tone CR tools free-of-charge.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.50). MS is the Chief Technology Officer at Clement Clarke International Limited, UK. WGA is an academic researcher in the field of inhaled respiratory medicine, inhaler technique and lung deposition. He has received unconditional travel grants from Clement Clarke International Limited to present his research findings at ATS, BTS, ERS and ISAM meetings. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research protocol was initially approved by the Research Ethics Committees at the Jordan University Hospital (Ref: 10/2015/1523) and at the Ministry of Health (Ref: MOH-REC-150131), Amman, Jordan. The study was conducted according to the Helsinki Declaration and Good Clinical Practice (ICH/GCP) Guidance and its updates. Eligible patients that agreed to take part signed an informed consent for this research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GINA. Global Initiative for Asthma Management and Prevention (GINA). 2019. Available online: . Accessed 01 June 2019.https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf
- Janson C, Johannessen A, Franklin K, et al. Change in the prevalence asthma, rhinitis and respiratory symptom over a 20 year period: associations to year of birth, life style and sleep related symptoms. BMC Pulm Med 2018;18:152. [Crossref] [PubMed]
- Makela MJ, Backer V, Hedegaard M, et al. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med 2013;107:1481-90. [Crossref] [PubMed]
- Dekhuijzen R, Lavorini F, Usmani OS, et al. Addressing the Impact and Unmet Needs of Nonadherence in Asthma and Chronic Obstructive Pulmonary Disease: Where Do We Go From Here? J Allergy Clin Immunol Pract 2018;6:785-93. [Crossref] [PubMed]
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax 2008;63:831-8. [Crossref] [PubMed]
- Bosnic-Anticevich SZ, Cvetkovski B, Azzi EA, et al. Identifying Critical Errors: Addressing Inhaler Technique in the Context of Asthma Management. Pulm Ther 2018;4:1-12. [Crossref] [PubMed]
- Price D, Bosnic-Anticevich S, Briggs A, et al. Inhaler competence in asthma: Common errors, barriers to use and recommended solutions. Respir Med 2013;107:37-46. [Crossref] [PubMed]
- Haughney J, Price D, Kaplan A, et al. Achieving asthma control in practice: Understanding the reasons for poor control. Respir Med 2008;102:1681-93. [Crossref] [PubMed]
- Al-Showair RA, Pearson SB, Chrystyn H. The Potential of a 2Tone Trainer To Help Patients Use Their Metered-Dose Inhalers. Chest 2007;131:1776-82. [Crossref] [PubMed]
- Crompton GK, Barnes PJ, Broeders M, et al. The need to improve inhalation technique in Europe: A report from the Aerosol Drug Management Improvement Team. Respir Med 2006;100:1479-94. [Crossref] [PubMed]
- Sanchis J, Gich I, Pedersen S. Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time? Chest 2016;150:394-406. [Crossref] [PubMed]
- Ammari WG, Al-Hyari N, Obeidat N, et al. Mastery of pMDI technique, asthma control and quality-of-life of children with asthma: A randomized controlled study comparing two inhaler technique training approaches. Pulm Pharmacol Ther 2017;43:46-54. [Crossref] [PubMed]
- Ammari WG, Chrystyn H. Optimizing the Inhalation Flow and Technique Through Metered Dose Inhalers of Asthmatic Adults and Children Attending a Community Pharmacy. J Asthma 2013;50:505-13. [Crossref] [PubMed]
- Azouz W, Campbell J, Stephenson J, et al. Improved Metered Dose Inhaler Technique When a Coordination Cap Is Used. J Aerosol Med Pulm Drug Deliv 2014;27:193-9. [Crossref] [PubMed]
- Griffith MF, Feemster LC, Donovan LM, et al. Poor Metered-Dose Inhaler Technique Is Associated with Overuse of Inhaled Corticosteroids in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc 2019;16:765-8. [Crossref] [PubMed]
- Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med 2017;27:22. [Crossref] [PubMed]
- Klijn SL, Hiligsmann M, Evers S, et al. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. NPJ Prim Care Respir Med 2017;27:24. [Crossref] [PubMed]
- Plaza V, Giner J, Rodrigo GJ, et al. Errors in the Use of Inhalers by Health Care Professionals: A Systematic Review. J Allergy Clin Immunol Pract 2018;6:987-95. [Crossref] [PubMed]
- Bonini M, Usmani OS. Novel methods for device and adherence monitoring in asthma. Curr Opin Pulm Med 2018;24:63-9. [Crossref] [PubMed]
- Juniper EF, O'Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902-7. [Crossref] [PubMed]
- Juniper EF, Bousquet J, Abetz L, et al. Identifying 'well-controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med 2006;100:616-21. [Crossref] [PubMed]
- Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J 1999;14:32-8. [Crossref] [PubMed]
- Virchow JC, Crompton GK, Dal Negro R, et al. Importance of inhaler devices in the management of airway disease. Respir Med 2008;102:10-9. [Crossref] [PubMed]
- Haughney J, Price D, Barnes NC, et al. Choosing inhaler devices for people with asthma: Current knowledge and outstanding research needs. Respir Med 2010;104:1237-45. [Crossref] [PubMed]
- Darquenne C, Fleming JS, Katz I, et al. Bridging the Gap Between Science and Clinical Efficacy: Physiology, Imaging, and Modeling of Aerosols in the Lung. J Aerosol Med Pulm Drug Deliv 2016;29:107-26. [Crossref] [PubMed]
- Kamin WES, Genz T, Roeder S, et al. Mass output and particle size distribution of glucocorticosteroids emitted from different inhalation devices depending on various inspiratory parameters. J Aerosol Med 2002;15:65-73. [Crossref] [PubMed]
- Farkas Á, Horváth A, Kerekes A, et al. Effect of delayed pMDI actuation on the lung deposition of a fixed-dose combination aerosol drug. Int J Pharm 2018;547:480-8. [Crossref] [PubMed]
- Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J 2002;19:246-51. [Crossref] [PubMed]
- Hindle M, Newton DAG, Chrystyn H. Investigations of an Optimal Inhaler Technique with the Use of Urinary Salbutamol Excretion as a Measure of Relative Bioavailability to the Lung. Thorax 1993;48:607-10. [Crossref] [PubMed]
- Newman S, Steed K, Hooper G, et al. Comparison of Gamma-Scintigraphy and a Pharmacokinetic Technique for Assessing Pulmonary Deposition of Terbutaline Sulfate Delivered by Pressurized Metered-Dose Inhaler. Pharm Res 1995;12:231-6. [Crossref] [PubMed]
- Biswas R, Hanania NA, Sabharwal A. Factors Determining In Vitro Lung Deposition of Albuterol Aerosol Delivered by Ventolin Metered-Dose Inhaler. J Aerosol Med Pulm Drug Deliv 2017;30:256-66. [Crossref] [PubMed]
- Farr SJ, Rowe AM, Rubsamen R, et al. Aerosol Deposition in the Human Lung Following Administration from a Microprocessor-Controlled Pressurized Metered-Dose Inhaler. Thorax 1995;50:639-44. [Crossref] [PubMed]
- Chrystyn H. Effects of Device Design on Patient Complience: Comparing the same Drug in Different Devices. RDD Eur 2009;1:105-16.
- Shim C, Williams MH. The Adequacy of Inhalation of Aerosol from Canister Nebulizers. Am J Med 1980;69:891-4. [Crossref] [PubMed]
- Burkhart PV, Rayens MK, Bowman RK. An evaluation of children's metered-dose inhaler technique for asthma medications. Nurs Clin North Am 2005;40:167-82. [Crossref] [PubMed]
- Tomlinson HS, Corlett SA, Allen MB, et al. Assessment of different methods of inhalation from salbutamol metered dose inhalers by urinary drug excretion and methacholine challenge. Br J Clin Pharmacol 2005;60:605-10. [PubMed]
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011;105:930-8. [Crossref] [PubMed]
- Ammari WG, Toor S, Chetcuti P, et al. Evaluation of asthma control, parents' quality of life and preference between AeroChamber Plus and AeroChamber Plus Flow-Vu spacers in young children with asthma. J Asthma 2015;52:301-7. [Crossref] [PubMed]
- Chen H, Gould MK, Blanc PD, et al. Asthma control, severity, and quality of life: Quantifying the effect of uncontrolled disease. J Allergy Clin Immunol 2007;120:396-402. [Crossref] [PubMed]
- Plaza V, Peiró M, Torrejón M, et al. A repeated short educational intervention improves asthma control and quality of life. Eur Respir J 2015;46:1298-307. [Crossref] [PubMed]



