Feasibility and prognostic benefit of induction chemoradiotherapy followed by surgery in patients with locally advanced non-small cell lung cancer
Introduction
Lung cancer remains the leading cause of cancer-related death in many countries, as many patients are diagnosed at an advanced stage (1). More than one third of all patients with non-small cell lung cancer (NSCLC) present with advanced disease at the time of the diagnosis (2). The standard treatment modality for patients with unresectable stage III NSCLC invading adjacent organs and/or cN2 is thought to be definitive chemoradiotherapy (3,4). However, the optimal treatment for patients with potentially “resectable” stage III NSCLC remains unclear. The standard therapy for patients with T3N0-1M0 NSCLC involving the chest wall is considered primary surgical resection. However, the prognosis of those patients has also been unsatisfactory (5,6).
Surgery alone results in a poor overall survival (OS) in patients with locally advanced NSCLC, such as lung cancer invading adjacent organs (invasive T3 or T4) and/or N2 lung cancer, as most such patients have microscopic distant metastases (7). Thus, the treatments for such advanced NSCLC should control both local and microscopic systemic disease. Multimodal treatment is therefore thought to be essential for improving the survival.
The administration of postoperative adjuvant chemotherapy is one approach to reduce the risk of recurrence. Adjuvant chemotherapy has been shown to significantly improve the survival in a meta-analysis and clinical trial (8,9); however, after undergoing major surgery, many patients are unable to receive adjuvant chemotherapy due to deterioration in their performance status (5). Another approach is to administer induction chemotherapy or chemoradiotherapy (ICRT) before the surgical procedure in order to control microscopic metastases and render the tumor completely resectable. Some analyses have demonstrated that ICRT improved the pathological complete response (CR) and local control rates in comparison to chemotherapy alone (10-14). ICRT has the potential to reduce the tumor size, achieve complete resection, eradicate micrometastases, and extract occult metastasis by performing a re-staging examination after ICRT (15).
The aim of this retrospective study was to analyze patients with resectable locally advanced NSCLC invading adjacent organs (invasive T3 or T4) and/or cN2 who underwent ICRT followed by surgery at our institute in order to evaluate the short- and long-term outcomes and investigate the relationship between the radiological/pathological response and survival.
Methods
The patient selection and evaluation
We retrospectively reviewed the clinical records of 84 patients with locally advanced NSCLC involving the adjacent structures or major vessels (invasive T3 or T4) and/or cN2 who underwent ICRT followed by surgery at Seirei Mikatahara General Hospital, Hamamatsu, Japan from December 2006 to May 2018. In order to avoid including false-positive cases, we only enrolled invasive T3/T4 patients in whom invasion of the surrounding structures invasion was pathologically confirmed after surgery. Patients with cN2 required radiological evidence of N2 disease, defined as both mediastinal nodal enlargement (short-axis diameter: >1 cm) on CT and an abnormal 18F-fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET) as well as pathological confirmation of the presence of N2 disease.
ICRT was performed for patients ≤75 years of age and with an Eastern Cooperative Oncology Group performance status of 0–1 and adequate organ functional reserves. In all cases, the tumor was potentially resectable with regression after ICRT. Patients were assessed by a thoracic surgeon, radiation oncologist, and medical oncologist before treatment. The disease stage was evaluated using chest radiography, enhanced chest and abdominal CT, enhanced brain magnetic resonance imaging (MRI), PET/CT, and bronchoscopy. The international Association of the Study of Lung Cancer TNM staging system for NSCLC, eighth edition, was used to determine the disease stage and nodal location (16).
Therapeutic schedule
In all cases, the chemotherapy regimens consisted of two courses of platinum doublet therapy. All patients underwent concurrent radiotherapy with 40 or 50 Gy to the primary tumor and involved lymph nodes. After ICRT, the patients were re-staged by thoracic CT, PET/CT, and brain MRI, and those without progressive disease (PD) were scheduled to receive radical surgery within six weeks of the completion of ICRT.
The surgical procedure employed was determined based on the extent of the tumor. Resection and reconstruction of the adjacent structures or major vessels was performed as necessary. The bronchial stump or anastomosis was basically covered with an intercostal muscle pedicle flap or pericardial fat tissue.
Postoperative adjuvant chemotherapy was administered to the greatest extent possible according to the pathological tumor response. If a pathological complete effect (Ef3) or major pathological effect (Ef2) was achieved by ICRT, the drugs that were administered for the induction regimen were administered for adjuvant chemotherapy. If no effect (Ef0), a minor effect (Ef1a), or mild effect (Ef1b) on pathological findings was observed, other platinum-based doublet agents were selected for adjuvant chemotherapy.
The patients were followed up every month until 12 weeks, every 3 months until 2 years after surgery, and every 6 months thereafter. Patients were scheduled to receive chest CT and brain MRI every 3 months and to receive abdominal CT, brain MRI, and a radionuclide bone scan or PET/CT every 12 months for at least 2 years. During the first 3–5 years after the completion of treatment, chest CT was repeated every 12 months.
The evaluation of the efficacy and toxicity
The pathologic tumor response (Ef) of induction therapy was assessed using resection specimens according to the General Rule for Clinical and Pathological Record of Lung Cancer (Eighth edition) by the Japan Lung Cancer Society (17): Ef0, no effect, no morphological changes including degeneration or necrosis caused by treatment; Ef1a, minor effect, viable cancer cells observed in two-thirds or more of cancer tissue; Ef.1b, mild effect, viable cancer cells observed in one-third or more but less than two-thirds of cancer tissue; Ef.2, moderate effect, viable cancer cells observed in less than one-third of cancer tissue; and Ef.3, marked effect, no viable cancer cells or residual cancer cells judged not to be viable. Complete resection (R0) was defined as resection with pathologic evidence of negative tumor margins.
Toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (version 4.0).
Statistical analyses
Categorical variables were analyzed by a χ2 test. The survival was estimated by the Kaplan-Meier method, and differences in the survival were determined by a log-rank analysis. Day zero was the date of pulmonary resection, and the final endpoint of the OS was death attributable to cancer, or due to a cause other than cancer. The final endpoint of the recurrence-free survival (RFS) was the date of recurrence, the date of the last follow-up examination, or the date of death in the absence of recurrence. Not all recurrent cases were confirmed pathologically. P values of less than 0.05 were considered to indicate statistical significance. All statistical analyses were performed using the StatView software program (SAS Institute Inc., Cary, NC, USA).
This retrospective analysis was approved by the Institutional Review Board of Seirei Mikatahara General Hospital (approval number: 18-30). The requirement for informed consent from each patient was waived owing to the retrospective nature of the study, with patient information obtained from the database.
Results
Patients’ characteristics
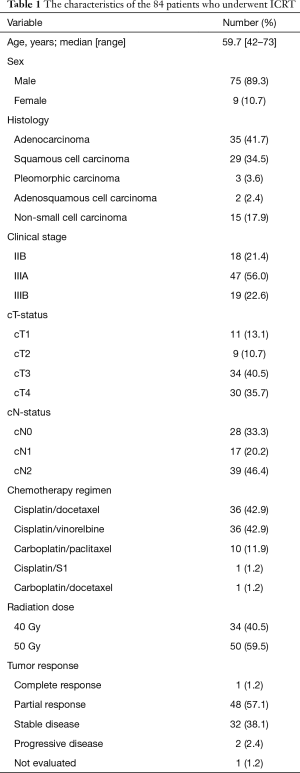
The characteristics of the patients are shown in Table 1. The median age was 59.7 years old (42–73 years old). There were 75 men and 9 women. The histological subtypes were as follows: adenocarcinoma (n=35), squamous cell carcinoma (n=29), non-small cell carcinoma (n=15), pleomorphic carcinoma (n=3), and adenosquamous cell carcinoma (n=2). Eighteen patients had stage IIB disease (T3N0), 47 patients had stage IIIA disease [T1N2 (n=11), T2N2 (n=9), T3N1 (n=6), T4N0 (n=10), and T4N1 (n=11)], and 19 patients had stage IIIB disease [T3N2 (n=10), T4N2 (n=9)]. In the clinical N2 patients, mediastinal nodal involvement was diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) (n=21) or CT/PET (n=18).
Full table
ICRT
The chemotherapy regimens of ICRT included cisplatin plus docetaxel (n=36), cisplatin plus vinorelbine (n=36), carboplatin plus paclitaxel (n=10), cisplatin plus S-1 (n=1), and carboplatin plus docetaxel (n=1). The radiation doses were 50 Gy (n=50) and 40 Gy (n=34).
The radiological responses to ICRT included a CR in 1 patient, a PR in 48, SD in 32, PD in 2 (both patients developed brain metastasis), and not evaluated due to death in 1; the overall response rate (ORR) was 59%.
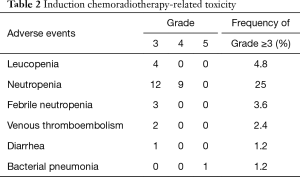
All 84 patients completed ICRT, which was generally well tolerated. Grade ≥3 adverse events are listed in Table 2. The most common grade 3 or 4 toxicity was neutropenia in 21 (25%) patients. One patient died after completing ICRT due to Nocardia pneumonia.
Full table
Surgery, pathological response, and postoperative adjuvant therapy
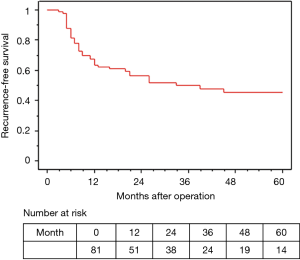
Surgery was performed in 81 (96%) patients, excluding 1 case of grade 5 pneumonia and 2 cases of PD (Figure 1).
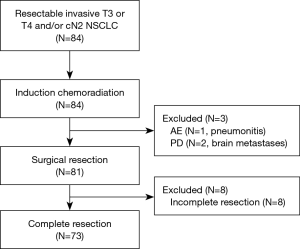
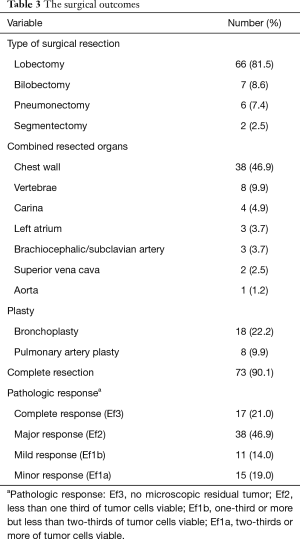
The surgical outcomes are shown in Table 3. The surgical procedure included lobectomy in 66 patients (81.5%), bilobectomy in 7 (8.6%), pneumonectomy in 6 (7.4%), and segmentectomy in 2 (2.5%). Tracheobronchoplasty was performed in 18 patients (22.2%); this included carinal resection in 4 and pulmonary arterioplasty in 8 (9.9%). Among the 6 pneumonectomies, 3 were right-sided (50%), and 2 involved carinal resections (33%). Combined resection was performed in 49 patients, involving the chest wall (n=38), vertebrae (n=8), left atrium (n=3), brachiocephalic/subclavian artery (n=3), superior vena cava (n=2), and aorta (n=1) (include duplicate patients). A total of 73 patients (90%) achieved complete resection (R0) (Figure 1).
Full table
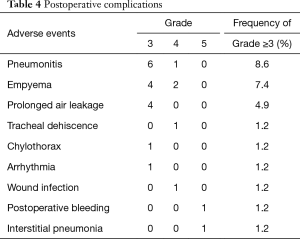
Grade ≥3 complications after surgery are listed in Table 4. The most common grade 3 or 4 toxicity was pneumonia in 7 (8.6%) patients. No intraoperative mortality was observed. Complication-related death within 30 postoperative days occurred in 1 patient (1.2%). The patient underwent left upper lobectomy combined with resection of the left subclavian artery and died eight days after surgery due to postoperative bleeding. Death after 30 postoperative days but within 90 days occurred in 1 patient (1.2%). The patient underwent right upper lobectomy combined with resection of carina and died 64 days after surgery due to interstitial pneumonia. One patient developed tracheal dehiscence after right wedge pneumonectomy combined with resection of the SVC, although the tracheal stump was buttressed using an intercostal muscle flap, and the patient was rescued by omentopexy. There were no significant differences in the mortality or morbidity rates of the patients who underwent lobectomy and pneumonectomy.
Full table
The therapeutic effect on the pathology was as follows: Ef3 (n=17; 21%), Ef2 (n=38; 47%), Ef1b (n=11; 14%), and Ef1a (n=15; 19%) (Table 3). Forty-three patients (51%) received adjuvant chemotherapy.
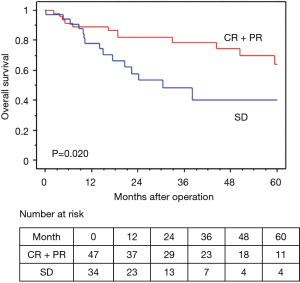
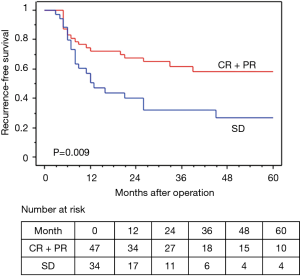
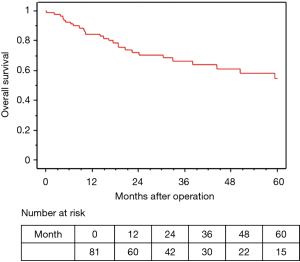
The median observation time was 37.2 months. The 5-year OS and RFS rates in all 81 surgically resected patients were 58.0% and 45.6%, respectively (Figures 2,3). The median survival time was 73.2 months. Among the 73 R0 patients, 26 developed recurrent disease during the follow-up period, and 21 of these 26 (81%) developed distant metastasis without loco-regional recurrence. Fourteen of the 17 patients with Ef3 were alive without recurrence, and 2 were alive with brain metastasis. The remaining patient was dead due to pneumonia at 9 months after surgery. Fifteen patients survived for more than 60 months after surgery.
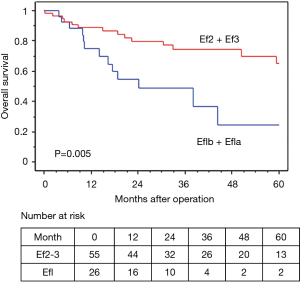
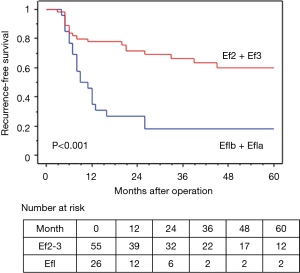
In terms of the prognosis of clinical stage, cStage IIB (cT3N0) disease showed a better 5-year OS than cStage IIIA and IIIB disease (62.7% vs. 54.9% vs. 50.3%), but there were no significant differences (P=0.33). However, based on the response to the ICRT, patients with a CR or PR showed a better 5-year OS and RFS than those with SD (OS: 63.7% vs. 40.0%, P=0.020, RFS: 58.4% vs. 26.7%, P=0.009) (Figures 4,5). Furthermore, patients with Ef3 or Ef2 exhibited a much better 5-year OS and RFS than those with Ef1b or Ef1a (OS: 65.0% vs. 24.4%, P=0.005, RFS: 60.1% vs. 17.9%, P<0.001) (Figures 6,7). There was no marked difference in the survival between patients who underwent lobectomy and those who underwent pneumonectomy (P=0.82). Patients who received adjuvant chemotherapy had a significantly better survival than those who did not receive adjuvant chemotherapy (72.2% vs. 36.7%, P=0.024).
Discussion
The data presented in the present study suggested that treatment with concurrent chemoradiotherapy followed by surgery might provide better local disease control and survival in patients with potentially resectable locally advanced NSCLC. Approximately half of the patients with resectable stage III (N2) NSCLC who received definitive chemoradiotherapy developed recurrence at a loco-regional site (18); thus, treatments that provide stronger local control, such as surgery, are necessary.
Invasive T3 or T4 locally advanced disease is associated with a risk of incomplete resection, especially when there is extensive invasion of important structures. Furthermore, many of these invasive T3 and T4 lung cancers have associated lymph node involvement. In this regard, the optimal treatment strategy for patients with potentially resectable locally advanced NSCLC is considered to be concurrent chemoradiotherapy followed by surgery. Some phase I and II studies demonstrated promising results for ICRT followed by surgery (18-24). However, some large-scale multi-institutional clinical trials comparing definitive chemoradiotherapy to ICRT followed by surgery for Stage III patients showed that adding surgery had no benefit (25,26). Albain et al. reported that the OS of patients who received ICRT with or without surgery did not differ to a statistically significant extent (INT0139) (25). However, the subset of patients who underwent lobectomy without pneumonectomy showed a significantly better survival in that trial. Furthermore, in their resected pT0N0 patients, an excellent median survival of 39.8 months was observed. Eberhardt et al. reported that patients who received ICRT followed by surgery tended to have a better survival than those who received definitive chemoradiotherapy (44% vs. 40%) (ESPATURE study) (26).
In terms of resectable invasive T3 NSCLC involving the chest wall, most thoracic surgeons recommend primary surgery followed by adjuvant therapy. However, the 5-year OS of those patients, even with N0 disease, has remained at 40–50% in the few previous studies (5,6). Therefore, induction therapy may be needed in order to improve their survival as well as that of stage III NSCLC. In their prospective study, Kawaguchi et al. showed that ICRT followed by surgery for patients with T3N0-1 NSCLC involving the chest wall was safe and effective, with a 5-year OS of 62.6% (27). In our study, the 5-year OS of the patients with T3N0-1 NSCLC involving the chest wall (n=23) reached 64.2%, which was better than the values reported in previous studies (5,6).
Our results demonstrated that a pathological response to ICRT was an important prognostic factor in the management of locally advanced NSCLC. When a good pathological response (Ef2 or Ef3) was achieved, a good prognosis could be expected. In various studies, a good pathological response determined a favorable survival and was associated with lower rates of local and distant recurrence as well as a favorable progression-free survival (28,29). Local tumor control and downstaging resulting in a tumor response and mediastinal lymph node clearance are potential surrogate endpoints for better patient outcomes. Achieving a good pathological response with ICRT is very important for improving the prognosis of patients with locally advanced NSCLC. In the present study, 17 (21%) patients achieved Ef3, which seems like a higher rate than in previous studies (25,30). The Ef3 rate of the patients receiving 50 Gy of radiation was higher than in those receiving 40 Gy of radiation (24.0% vs. 17.6%). Therefore, higher-dose radiotherapy with concurrent chemotherapy may provide a greater pathologic effect. In terms of tumor histology, a significantly higher percentage of patients with squamous cell carcinoma had Ef2 or Ef3 than those with non-squamous cell carcinoma (85.1% vs. 59.3%, P=0.018). Similar results have been reported previously (12). The mechanism underlying this result is unclear, however, and further investigation is required.
The surgical treatment of patients with locally advanced lung cancer remains a challenge. Chemoradiotherapy before surgery has been associated with a higher risk of intraoperative and postoperative complications. In a previous study, the incidence of surgical mortality after ICRT was reported to be high (15,18-20). However, the mortality rate in our study (1.2%) was lower than in previous studies. Recent studies have also reported low mortality rates; therefore, ICRT followed by surgery is thought to be feasible (13,14,30-32). In the INT0139 study, the patients who underwent pneumonectomy showed higher rates of surgical mortality (26%) and a worse prognosis than those who underwent lobectomy. However, Weder et al. reported that the 90-day mortality rate of 176 patients who underwent induction chemotherapy or chemoradiotherapy followed by pneumonectomy was only 3% in a retrospective evaluation of medical records at two specialized centers (33). Similarly, the mortality/morbidity and survival rates of the patients who underwent pneumonectomy and lobectomy in our study did not differ to a statistically significant extent. While pneumonectomy should be avoided by performing tracheobronchoplasty and/or arterioplasty whenever possible, pneumonectomy after ICRT is thought to be a valuable treatment option for achieving complete resection.
The present study was associated with several limitations. First, this study was retrospective in nature and lacked randomization. Second, the study population was relatively small. The fact that this was performed in a single institution may limit the generalizability of the findings. Finally, the study period was long, and various chemotherapy regimens were administered. We are currently performing a single-institutional phase II study of ICRT followed by surgery for patients with stage III NSCLC.
In conclusion, our results suggested that a favorable prognosis can be expected when CR/PR or Ef2/Ef3 is achieved by ICRT. ICRT significantly improved the survival of patients with locally advanced lung cancer. In experienced centers, surgery after ICRT can be safely performed without significant mortality.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective analysis was approved by the Institutional Review Board of Seirei Mikatahara General Hospital (approval number: 18-30).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin 2012;62:220-41. [Crossref] [PubMed]
- Ramanath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S-340S.
- Bezjak A, Temin S, Franklin G, et al. Defenitive and adjuvant radiotherapy in locally advanced non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. J Clin Oncol 2015;33:2100-5. [Crossref] [PubMed]
- Kawaguchi K, Miyaoka E, Asamura H, et al. Modern surgical results of lung cancer involving neighboring structures: a retrospective analysis of 531 pT3 cases in a Japanese Lung Cancer Registry Study. J Thorac Cardiovasc Surg 2012;144:431-7. [Crossref] [PubMed]
- Doddoli C, D'Journo B, Le Pimpec-Barthes F, et al. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg 2005;80:2032-40. [Crossref] [PubMed]
- Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung cancer 1998;21:1-6. [Crossref] [PubMed]
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552-9. [Crossref] [PubMed]
- Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995;13:1880-92. [Crossref] [PubMed]
- Jaklitsch MT, Herndon JE 2nd, DeCamp MM Jr, et al. Nodal downstaging predicts survival following induction chemotherapy for stage IIIA (N2) non-small cell lung cancer in CALGB protocol #8935. J Surg Oncol 2006;94:599-606. [Crossref] [PubMed]
- Chen F, Okubo K, Sonobe M, et al. Hyperfractionated irradiation with 3 cycles of induction chemotherapy in stage IIIA-N2 lung cancer. World J Surg 2012;36:2858-64. [Crossref] [PubMed]
- Toyooka S, Kiura K, Shien K, et al. Induction chemoradiotherapy is superior to induction chemotherapy for the survival of non-small-cell lung cancer patients with pathological mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg 2012;15:954-60. [Crossref] [PubMed]
- Katakami N, Tada H, Mitsudomi T, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012;118:6126-35. [Crossref] [PubMed]
- Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med 2004;350:379-92. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- The Japan Lung Cancer Society. General rule for clinical and pathological record of lung cancer, 8th edition. Tokyo: Kanehara & Co, 2017.
- Darling GE, Li F, Patsios D, Massey C, et al. Neoadjuvant chemoradiation and surgery improves survival outcomes compared with definitive chemoradiation in the treatment of stage IIIA N2 non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;48:684-90. [Crossref] [PubMed]
- Choi NC, Carey RW, Daly W, et al. Potential impact on survival of improved tumor downstaging and resection rate by preoperative twice-daily radiation and concurrent chemotherapy in stage IIIA non-small-cell lung cancer. J Clin Oncol 1997;15:712-22. [Crossref] [PubMed]
- Katakami N, Okazaki M, Nishiuchi S, et al. Induction chemoradiotherapy for advanced stage III non-small cell lung cancer: long-term follow-up in 42 patients. Lung Cancer 1998;22:127-37. [Crossref] [PubMed]
- Eberhardt W, Wilke H, Stamatis G, et al. Preoperative chemotherapy followed by concurrent chemoradiation therapy based on hyperfractionated accelerated radiotherapy and definitive surgery in locally advanced non-small-cell lung cancer: mature results of a phase II trial. J Clin Oncol 1998;16:622-34. [Crossref] [PubMed]
- Daly BD, Ebright MI, Walkey AJ, et al. Impact of neoadjuvant chemoradiotherapy followed by surgical resection on node-negative T3 and T4 non-small cell lung cancer. J Thorac Cardiovasc Surg 2011;141:1392-7. [Crossref] [PubMed]
- Shien K, Toyooka S, Kiura K, et al. Induction chemoradiotherapy followed by surgical resection for clinical T3 or T4 locally advanced non-small cell lung cancer. Ann Surg Oncol 2012;19:2685-92. [Crossref] [PubMed]
- Tanaka F, Yokomise H, Soejima T, et al. Induction Chemoradiotherapy (50 Gy), Followed by Resection, for Stage IIIA-N2 Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;106:1018-24. [Crossref] [PubMed]
- Albain KS, Swann RS, Rush VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomized controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Eberhardt WE, Pöttgen C, Gauler TC, et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194-201. [Crossref] [PubMed]
- Kawaguchi K, Yokoi K, Niwa H, et al. A prospective, multi-institutional phase II study of induction chemoradiotherapy followed by surgery in patients with non-small cell lung cancer involving the chest wall (CJLSG0801). Lung Cancer 2017;104:79-84. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]
- Schreiner W, Gavrychenkova S, Dudek W, et al. Pathologic complete response after induction therapy-the role of surgery in stage IIIA/B locally advanced non-small cell lung cancer. J Thorac Dis 2018;10:2795-803. [Crossref] [PubMed]
- Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049-56. [Crossref] [PubMed]
- Girard N, Mornex F, Douillard JY, et al. Is neoadjuvant chemoradiotherapy a feasible strategy for stage IIIA-N2 non-small cell lung cancer? Mature results of the randomized IFCT-0101 phase II trial. Lung Cancer 2010;69:86-93. [Crossref] [PubMed]
- Yang CF, Gulack BC, Gu L, et al. Adding radiation to induction chemotherapy does not improve survival of patients with operable clinical N2 non-small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:1484-92; discussion 1492-3. [Crossref] [PubMed]
- Weder W, Collaund S, Eberhardt WE, et al. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J Thorac Cardiovasc Surg 2010;139:1424-30. [Crossref] [PubMed]