
Chimeric antigen receptor T cell therapies for thoracic cancers—challenges and opportunities
It is widely accepted that the immune system plays a critical role in controlling cancer. Immunotherapies exploit this complex interplay by activating the immune response to target and clear cancer cells. Chimeric antigen receptor (CAR) T cells are genetically engineered T cells that target and specifically recognise tumour antigens and have demonstrated curative responses in certain blood cancers (1,2). Currently CAR T cells have been approved by the Food and Drug for the treatment of certain B cell malignancies with rapidly increasing interest in solid tumours (3). This editorial will highlight CAR T cell design, therapeutic strategies and potential roadblocks to the application of CAR T cells for the treatment of thoracic cancers.
Adoptive cell transfer (ACT) based cancer therapy
Meta-analysis studies have demonstrated that elevated levels of tumour infiltrating lymphocytes (TILs) are often correlated with prolonged patient survival (4). Adoptive T cell transfer therapy (ACT) is a cell-based therapy that aims to increase the number of tumour specific immune T cells in cancer patients. Here, TILs are isolated, activated ex vivo, and adoptively transferred back into patients in order to facilitate improved patient outcomes (5). However, responses to TIL-based ACT have only demonstrated efficacy in certain cancers, such as melanoma, and can vary greatly between patients (6). Furthermore, it is not always possible to isolate and expand TILs from every patient (5). To circumvent these issues, methods for transducing the first CAR expressing T cells were developed in the early 1990s (7,8).
CAR T cell therapy
CAR T cells are generated from peripheral blood lymphocytes. Patient T cells are transduced ex vivo to express CARs cognate for tumour antigens, thereby directing T cells to specifically kill tumour cells (5). A CAR is composed of an antigen-specific derived, single-chain variable fragment (scFv) linked to intracellular signalling domains (8) (Figure 1). Direct recognition of cancer antigens through the scFv facilitates T cell activation and tumour cell killing without the requirement for tumour antigen presentation through the major histocompatibility complex. The first developed CAR T cells were engineered to include a single intracellular signalling domain such as CD3-ζ (8) (Figure 1). Second- and third-generation CAR T cells introduced additional intracellular co-stimulation signalling domains to achieve more efficacious CAR T cell activation and greater in vivo persistence (9,10) (Figure 1).
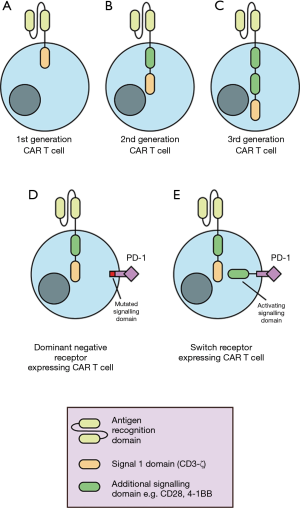
The success of CAR T cell treatment has resulted in FDA approval for the use of two CD19-targeted CAR T cell therapies in 2017. These included tisagenlecleucel (KYMRIAH) for the treatment of children and adolescent’s acute lymphoblastic leukemia (ALL) and axicabtagene ciloleucel (YESCARTA) for adult relapsed-refractory large B-cell lymphoma (3). More recently, the FDA has provided its regenerative medicine advanced therapy designation to CAR T cell treatment of relapsed or refractory multiple myeloma also known as CT053. Favourable outcomes in these malignancies have led to the investigation of CAR T cell treatment in the context of a range of thoracic cancers.
The challenges of CAR T cell therapy
Despite favourable outcomes in the treatment of haematological malignancies, CAR T-cells targeting solid tumours have demonstrated inadequate efficacy in thoracic cancers (10,11). This is believed largely attributed to poor trafficking of CAR T cells to the tumour site, the immunosuppressive tumour microenvironment (TME), poor activation and persistence of CAR T cells in vivo (12).
Inadequate lymphocyte recruitment to the tumour may be due to various factors including aberrant tumour vasculature, endothelial anergy, and mismatch of the TME chemokine profile and CAR T cell chemokine receptors (13). CAR T cell exclusion can be overcome through transduction of T cells to overexpress the relevant chemokine receptors in addition to a CAR. This has been demonstrated in the treatment of malignant pleural mesothelioma (MPM), which has a high level of CCL2 chemokine secretion (14). The overexpression of chemokine receptor 2 (CCR2) by mesothelin (MSLN) specific CAR T cells improved pleural accumulation and anti-tumour activity of CAR T cells against MPM (15). Furthermore, chemokine exclusion of CAR T cells may be overcome through regional delivery of CAR T cells to appropriate sites, as opposed to intravenous infusion. Improved CAR T cell persistence and anti-tumour activity has been demonstrated in regional delivery to orthotopic MPM mouse tumours and is now being evaluated in phase I clinical trials (16).
In addition to inhibition of trafficking, CAR T cells must overcome a wide variety of secreted and cellular factors in the TME which further act to suppress T cell killing activity. These TME factors include immunosuppressive cells, inhibitory ligands and receptors, and immunosuppressive factors. These challenges constitute some of the biggest barriers to successful CAR T cell treatment of thoracic cancers. The generation and recruitment of immunosuppressive cells to the TME is a key characteristic of a developing tumour (17). Major immunosuppressive subsets associated with lung cancer and mesothelioma include myeloid derived suppressor cells (MDSCs), mesenchymal stromal cells (MSCs), tumour-associated macrophages (TAMs) and regulatory T cells (Tregs) (18). Whilst CAR T cells targeting immunosuppressive subsets have been generated and utilised in pre-clinical studies, such cells are yet to be tested in the clinical setting.
Another strategy to target the TME is to block immune checkpoint receptor-ligand interactions. Immune checkpoint receptors are naturally expressed by activated T cells and function to minimise collateral host tissue damage during the immune response. Immunosuppressive myeloid populations and tumour cells may both aberrantly express ligands for immune checkpoint receptors expressed by endogenous and CAR T cells, particularly, ligands for programmed death-1 (PD-1). Ligation of immune checkpoint receptors suppresses CAR T cell anti-tumour function and polarises CAR T cells to a state of functional exhaustion (19). Disruption of immune checkpoint receptor-ligand interactions has been a successful treatment strategy for solid tumours. Antagonistic, monoclonal antibodies against CTLA-4, PD-1, and programmed death ligand 1 (PD-L1) have been FDA approved for the treatment of range of cancers, including non-small cell lung cancer (NSCLC) (20). Therefore, the combination of CAR T cells with checkpoint inhibitors has great potential in treating patients with thoracic cancers. In fact, in pre-clinical studies, the combination of CAR T cell and immune checkpoint blockade therapies has demonstrated significant efficacy in syngeneic mouse models, driving significant tumour regression, improved survival and prevented CAR T cell exhaustion (21).
Another approach for immune checkpoint receptor inhibition is the modification of CAR T cells to express dominant negative receptors (DNRs) or switch receptors. DNRs are mutated receptors for immunosuppressive signals in the TME that abrogate signalling and negative regulation. DNRs generated for PD-1 have demonstrated increased CAR T cell resistance to immunosuppression and restored effector function (22). Moreover, CAR T cell DNRs can also can also compete with endogenous T cells expressing immune checkpoint receptors of endogenous T cells, reducing the inhibition of endogenous anti-tumour T cell responses. Conversely, switch receptors are composed of an extracellular antigen binding portion of an antibody specific for immunosuppressive molecules such as PD-1 or CTLA-4 conjugated to intracellular activation signalling domains (12). Expression of switch receptors against CTLA-4 has shown to increase T cell efficacy in mouse models (23). In summary, combination therapy of CAR T cells with different approaches to immune checkpoint receptor inhibition may prove to be a promising avenue for the treatment of a range of thoracic cancers.
As CAR T cells are antigen specific, tumour heterogeneity has proven to be one the of the greatest hurdles for effective CAR T cell therapy in the solid tumour settings. It is considered that recruitment of the endogenous anti-tumour response following CAR T cell activity is required for broad protection against solid tumours (24). In addition to immune checkpoint blockade that may rescue previously inhibited endogenous immune cell types, modified ‘armoured’ CAR T cells can also recruit endogenous immune components. Armoured CAR T cells are engineered to express molecular factors that may facilitate immune cell activation and recruitment. For example, armoured CAR T cells modified to express single chain IL-12 have demonstrated elevated efficacy compared to conventional CAR T cell therapy in xenograft models (25). The constitutive IL-12 signalling provided through armoured CAR T cells enhanced T cell cytotoxicity, cytokine secretion and resistance against Treg immunosuppression (25). However, armoured-CAR activity can cause severe cytokine release syndrome (CRS) and has yet to demonstrate safety and efficacy in clinical trials.
Clinical investigation of CAR T cell treatment for thoracic cancers
Whilst effective CAR T cell therapy requires an antigen target that has high tumour expression, solid tumour antigens targeted by CARs are often also expressed on normal, healthy tissue (26). This raises significant risks for off-target effects, where toxicity has been demonstrated in a number of CAR T cell clinical trials (26). A selection of clinical trials studying CAR T cells in thoracic cancer are listed in Table 1.
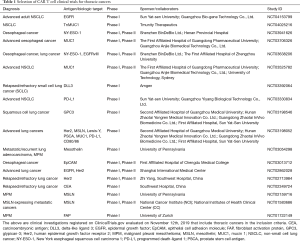
Full table
The focus of research for the field remains in the discovery of tumour associated or tumour specific target antigens. Human epidermal growth factor receptor 2 (Her2) and epidermal growth factor receptor (EGFR) have presented as popular solid tumour targets in both pre-clinical and clinical CAR T cell investigations in thoracic cancers. Her2 and EGFR belong to the ErbB family of receptor tyrosine kinases and are often overexpressed in a range of thoracic tumour contexts (27). Phase I clinical trials of CAR T cells targeting EGFR in relapsed/refractory NSCLC demonstrated patient tolerance to therapy, CAR T cell tumour infiltration and depletion of EGFR expressing tumour cells in tumour biopsy samples (28).
MSLN is normally expressed by mesothelial cells of the pleura, peritoneum and pericardium, but can have increased expression in MPM and lung adenocarcinoma cases (29). Advanced MPM patients in a phase I clinical trial that received MSLN targeted CAR T cells demonstrated no obvious off-tumour on-target toxicity, and trafficking to primary and metastatic tumour sites. Moreover, tumour cell lysis and the decrease in tumour cell numbers in ascites was suggested to be caused by CAR T cell killing (11).
Pre-clinical studies in mouse NSCLC xenograft models targeting prostate stem cell antigen (PSCA) and Mucin 1 (MUC1) have shown that dual therapy of CAR T cells targeting PSCA and MUC1 can synergistically eliminate tumours co-expressing both target antigens (30). Of note, MUC1 is a glycoprotein expressed by epithelial cells on mucosal surfaces and is aberrantly expressed in NSCLC and lung adenocarcinomas. Currently there are a number of active clinical trials involving MUC1 directed CAR T cells for the treatment of thoracic cancers (NCT03706326 and NCT03525782).
CAR T cells targeting PD-L1 expressed by immunosuppressive cells and tumour cells present as an interesting opportunity to eliminate both tumour cells and mechanisms of immunosuppression. Although clinical and pre-clinical data supports the use of checkpoint blockade antibodies or the use of CAR T cells with intrinsic resistance to PD-1 checkpoint blockade, the benefit of CAR T cells specifically targeting PD-L1 has yet to be elucidated. There are currently a number of clinical trials studying the efficacy of PD-L1 targeting CAR T cells in thoracic cancers (NCT03198052, NCT03330834 and NCT03060343).
Summary and future perspectives
With the clinical landscape for CAR T cell treatment of haematological malignancies becoming increasingly well defined, further understanding of the mechanisms by which the TME suppresses CAR T cells will be critical in shaping the success of CAR T cell treatment in thoracic malignancies. Moreover, immunosuppressive mechanisms, tumour antigen expression, metastasis and tumour cell metabolism can differ greatly between tumour types. As such, a modular system in which CAR T cells can be paired with the correct CAR, chemokine sensitivity and resistance to immunosuppression will be key in providing effective, patient specific care in thoracic cancers.
Acknowledgments
Funding: This work was supported by grants from the Peter MacCallum Cancer Centre Foundation, the National Health and Medical Research Council (NHMRC) of Australia (1176935, 1103352 and 1132373), the National Breast Cancer Foundation (NBCF) of Australia (IIRS-18-064 and IIRS-20-073) and Susan G. Komen Breast Cancer Foundation (16376637). JDC was supported by a Research Training Program (RTP) and Rosie Lew scholarships. PKD and MHK were supported by NHMRC Senior Research Fellowships. CYS was supported by a Postdoctoral Fellowship from the NBCF.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Peng Luo, Clare Y. Slaney and Jian Zhang) for the series “Immunotherapy and Tumor Microenvironment” published in Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.34). The series “Immunotherapy and Tumor Microenvironment” was commissioned by the editorial office without any funding or sponsorship. CYS served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010;116:4099-102. [Crossref] [PubMed]
- Slaney CY, Wang P, Darcy PK, et al. CARs versus BiTEs: A Comparison between T Cell-Redirection Strategies for Cancer Treatment. Cancer Discov 2018;8:924-34. [Crossref] [PubMed]
- Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93-103. [Crossref] [PubMed]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62-8. [Crossref] [PubMed]
- Kershaw MH, Teng MWL, Smyth MJ, et al. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol 2005;5:928-40. [Crossref] [PubMed]
- Goverman J, Gomez SM, Segesman KD, et al. Chimeric immunoglobulin-T cell receptor proteins form functional receptors: implications for T cell receptor complex formation and activation. Cell 1990;60:929-39. [Crossref] [PubMed]
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989;86:10024-8. [Crossref] [PubMed]
- Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004;18:676-84. [Crossref] [PubMed]
- Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013;13:525-41. [Crossref] [PubMed]
- Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2014;2:112-20. [Crossref] [PubMed]
- Yong CSM, Dardalhon V, Devaud C, et al. CAR T-cell therapy of solid tumors. Immunol Cell Biol 2017;95:356-63. [Crossref] [PubMed]
- Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res 2014;74:7168-74. [Crossref] [PubMed]
- Chene AL, d'Almeida S, Blondy T, et al. Pleural Effusions from Patients with Mesothelioma Induce Recruitment of Monocytes and Their Differentiation into M2 Macrophages. J Thorac Oncol 2016;11:1765-73. [Crossref] [PubMed]
- Moon EK, Carpenito C, Sun J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res 2011;17:4719-30. [Crossref] [PubMed]
- Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 2014;6:261ra151. [Crossref] [PubMed]
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 2007;25:267-96. [Crossref] [PubMed]
- Aerts JG, Lievense LA, Hoogsteden HC, et al. Immunotherapy prospects in the treatment of lung cancer and mesothelioma. Transl Lung Cancer Res 2014;3:34-45. [PubMed]
- John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology 2013;2:e26286. [Crossref] [PubMed]
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 2018;8:1069-86. [Crossref] [PubMed]
- John LB, Devaud C, Duong CP, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res 2013;19:5636-46. [Crossref] [PubMed]
- Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 2016;126:3130-44. [Crossref] [PubMed]
- Shin JH, Park HB, Oh YM, et al. Positive conversion of negative signaling of CTLA4 potentiates antitumor efficacy of adoptive T-cell therapy in murine tumor models. Blood 2012;119:5678-87. [Crossref] [PubMed]
- Jackson HJ, Brentjens RJ. Overcoming Antigen Escape with CAR T-cell Therapy. Cancer Discov 2015;5:1238-40. [Crossref] [PubMed]
- Zhang L, Kerkar SP, Yu Z, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther 2011;19:751-9. [Crossref] [PubMed]
- Ali AI, Oliver AJ, Samiei T, et al. Genetic Redirection of T Cells for the Treatment of Pancreatic Cancer. Front Oncol 2019;9:56. [Crossref] [PubMed]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127-37. [Crossref] [PubMed]
- Feng K, Guo Y, Dai H, et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci 2016;59:468-79. [Crossref] [PubMed]
- Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer 2008;44:46-53. [Crossref] [PubMed]
- Wei X, Lai Y, Li J, et al. PSCA and MUC1 in non-small-cell lung cancer as targets of chimeric antigen receptor T cells. Oncoimmunology 2017;6:e1284722. [Crossref] [PubMed]


