
miR-107 inhibited malignant biological behavior of non-small cell lung cancer cells by regulating the STK33/ERK signaling pathway in vivo and vitro
Introduction
Non-small cell lung cancer (NSCLC), a kind of lung cancer, is one of the most prominent contributors to global cancer-related mortality rates due to its high prevalence, late diagnosis, aggressiveness, lack of effective treatment, and poor prognosis (1). The malignancy of NSCLC means there is an urgent need for new Biomarker strategies which enable early detection and treatment of NSCLC to be discovered.
Extensive cancer research studies have explored the relationship between miRNAs as small non-coding RNAs and proliferation, migration, invasion, and drug resistance in many varieties of cancer (2). A number of NSCLC-focused studies have found that miRNAs could also regulate the biological behavior of NSCLC (3). One study, for example, reported that miR-221 serves an oncogenic role in NSCLC (4). In addition, miR-608 has been found to promote apoptosis in NSCLC (5). Moreover, miR-330-3p promotes epithelial-mesenchymal transition and brain metastasis in NSCLC (6). Among all the miRNAs related to NSCLC development, miR-107 plays an important role. For example, one study reported that miR-107 suppressed the growth of NSCLC cell lines by inducing cell cycle arrest (7). In addition, miR-107 inhibits tumor growth and metastasis in NSCLC (8). Nevertheless, the mechanism of the effect of miR-107 in NSCLC is worthy of further study.
Of all protein-coding RNAs, more than half are thought to be subordinated to miRNA-mediated control (9). In our previous studies, miRNAs were seen to regulate the biological behavior of cancer by targeting mRNAs. In addition, with the aid of bioinformatics tools, we established that STK33 is one of the target genes of miR-107. Moreover, another study reported that STK33 was related to the development of NSCLC (10). Furthermore, extracellular signal-regulated kinase 2 (ERK2) was established as a substrate of STK33 (11), while the relation between the ERK signaling pathway and the development of NSCLC have been proved (12). In this study, we examined the expression level of miR-107 in NSCLC cell lines and tissues, then verified the effect miR-107 had on the malignant biological behavior of NSCLC in two NSCLC cell lines in mice. Finally we researched the mechanism of miR-107 regulated cell proliferation, apoptosis, invasion, and tumor growth of NSCLC. This study aimed to provide a reliable theory for NSCLC gene therapy.
Methods
Tissue samples
We collected NSCLC tissues from 20 patients treated in the Department of Thoracic Surgery I at The Third Affiliated Hospital of Kunming Medical University. None had received radiotherapy or chemotherapy prior to surgery. All the patients signed informed consent.
Cell culture and transfection
Normal lung epithelial cells (Beas-2B, #XK-XB-2140) and NSCLC cells (A549, #XK-XB-1148; H1299, #XK-XB-1296; H520, #XK-XB-1313; and H1975, #XK-XB-1983) were obtained from Shanghai Xuanke Biotechnology Co., Ltd. (Shanghai, China). Cell lines were cultured using Dulbecco’s Modified Eagle Medium (DMEM) (#CM0324, LEAGEN, Shanghai, China) with 10% FBS (#01-0136-00, merck-millipore, USA) in an incubator at standard culture conditions, 37 °C and 5% CO2. miR-107 mimics, miR-107 inhibitor, and si-STK33 were synthesized by Guangzhou Ribo Biotechnology Co., Ltd. (China) and transfected into A549 and H1975 using Lipofectamine 2000 (#DXT-11668019, Gibco, USA) in adherence with the manufacturer’s instructions.
RT-qPCR
The expression levels of RNA were determined by RT-qPCR. TRIZOL reagent (#DXT-15596018, Gibco, USA) was used to extract total RNA from all of the cell lines and tissues named above, before it was transcribed into cDNA with PrimeScript™ RT reagent Kit (#RR037A, Takara Bio, Shiga, Japan). Polymerase chain reaction was carried out using the TB Green Premix Ex Taq II. The thermocycling profiles were as follows: 95 °C for 3 min, followed by 40 cycles of 95 °C for 12 s, and then 62 °C for 40 s. Relative gene expressions were calculated by 2−△△CT method relative to U6. The primer sequences were as follows: miR-107 forward primer: 5'-ATGATGAGCAGCATTGTACAGG-3', and reverse primer: 5'-GCAGGGTCCGAGGTATTC-3'. STK33 forward primer: 5'-GTGCGTATTTGTCGGAGATTC-3', and reverse primer: 5'-TACCATAACAACGACCTAACCG-3'. U6 forward primer: 5'-CTCGCTTCGGCAGCACA-3', and reverse primer: 5'-AACGCTTCACGAATTTGCGT-3.
Western blotting
Protein expression was detected using Western blotting. Harvesting of the cell lines took place 48 h after they were transfected. The extraction of total proteins was performed with RIPA assay lysis buffer (#2114-100, BioVision, USA). Total protein concentration was measured with a BCA Protein Assay kit (#orb-EHJ033662, BIOHJSW, USA). SDS-PAGE was used for the separation of proteins and then transferral onto a PVDF membrane, which was then subjected to blocking with 5% non-fat milk at room temperature for 1 h, before incubation with the primary antibody STK33 (1:1,000 dilution, #FNab08334, FineTest, Wuhan, China), ERK2 (1:1,000 dilution, #abx010745, Abbexa, UK), p-ERK2 (1:1,000 dilution, #p42 MAP Kinase (Erk2) Antibody, CST, USA) and c-Fos (1:1,000 dilution, #GTX50848, GeneTex, USA) at 4 °C for 12 h. The next day, the membranes underwent a period of incubation with horseradish peroxidase-conjugated secondary antibody (1:1,000 dilution, #31001, PIERCE, USA) for 2 h at room temperature. Enhanced chemiluminescence reagent (#131023-60-4, BOC Sciences, USA) enabled the visualization of the bands, and analysis was conducted using ImageJ software.
CCK-8
Cell proliferation was determined with cell counting kit-8 (CCK-8, #E1CK-00208, Enogene, Nanjing, China) assay. Cells were first seeded in 96-well plates before the addition of CCK-8 to each well at 0, 24, 48, 72, and 96 h. After being incubated over a period of 4 h, the culture media containing the CCK-8 was removed. A microplate reader was used to measure the absorbance (OD) at 450 nm wavelength.
Transwell assay
Cell invasive ability was evaluated by Transwell assay. The cells were seeded in the Matrigel (#M8370, Solarbio, Shanghai, China)-coated upper chamber, and the lower chambers were loaded with normal medium. After incubation lasting 48 h, a swab was used for the removal of the cells which had not invaded, and the cells which had invaded through to the lower surface were fixed with 4% methanol (#JKLN042113, Jingke, Shanghai, China), followed by staining with 0.1% crystal violet (#JKLN016612, Jingke, Shanghai, China). Five fields were randomly selected for cell count, observation, and photography.
Flow cytometry
Cell apoptosis levels were determined by flow cytometry for all of the groups. Cells were first seeded on 6-well plates, before being incubated for 24 h along with Annexin V-FITC/PI Apoptosis Kit (#KA3805, abnova, Shanghai, China), and Analysis using flow cytometry. The apoptotic cells showed a positive Annexin V-FITC signal and a negative PI signal. FlowJo software version determined the number of cells at each phase.
Dual-Luciferase reporter gene
The miR-107 and STK33 3'UTR binding sites were predicted using starBase. pGL3-STK33 WT or pGL3-STK33 Mut synthesized by Wuhan Yipu Biotechnology Co., Ltd. (China) was transfected along with NC or miR-107 mimics into 293T cells. After transfection for 48 h, the luciferase activity of all the groups was measured by Dual-Luciferase Assay System.
In vivo tumor growth assay
To investigate the effects of miR107, STK33, and the ERK signaling pathway on tumorigenicity of NSCLC, the indicated cells, A549 and H1975, were infected with lentivirus. After infection for 48 h, the A549 and H1975 were collected and implanted subcutaneously into nude mice. The tumor volume was calculated with the following formula: length × width2 × 0.5, and was measured at day 4, 7, 11, 15, 19, 23, and 27 after implantation. The mice were sacrificed on 28 days after injection, the tumors were removed and their weights measured. All experiments involving animals in this study received approval from the Experimental Animal Ethical Committee of the Department of Thoracic Surgery I at The Third Affiliated Hospital of Kunming Medical University.
Immunohistochemistry staining
The tumors removed from the mice were embedded with paraffin and fixed with formalin. Primary antibodies STK33 (#PAab08334, LSM Bio, Wuhan, China) and p-ERK2 (#LFMA0179, Pierce, Shanghai, China) were used for staining. Then, HRP-conjugated secondary antibodies (#EB 1, Detroit R&D, USA) were used, and the signal was visualized using 3,3'-Diaminobenzidine (DAB, #40470006-3, Bio-world, USA).
Statistical analyses
All experiments in this study were performed in triplicate. All the data were presented as the mean ± SD deviation of the three repeated experiments. Depending on the comparison being made, either t-test or one-way ANOVA was performed to assess statistical significance between two groups. SPSS 22.0 calculated the data, and figures were constructed with Graphpad Prism 5.0. A statistically significant difference was indicated when P<0.05.
Results
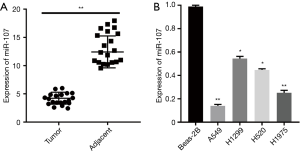
miR-107 was downregulated in NSCLC cell lines and tissues
Dysregulation of miRNAs is a common mechanism in the development of lung cancer, thus we measured the levels of miR-107 expression in NSCLC tissues and cell lines. The RT-qPCR results revealed miR-107 to be remarkably lower in NSCLC tissues compared with adjacent tissues (Figure 1A). Furthermore, compared with normal lung epithelial cell lines, NSCLC cell lines had significantly downregulated miR-107 expression, especially A549 and H1975 (Figure 1B). Therefore, miR-107 was downregulated in NSCLC tissues and cell lines. Based on our results, two NSCLC cell lines, A549 and H1975, were selected for additional experiments.
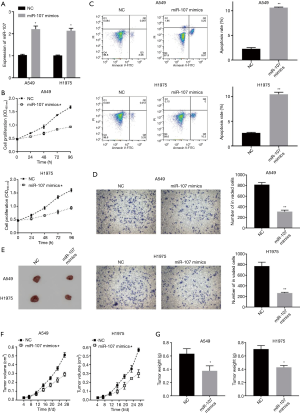
miR-107 overexpression inhibited the malignant biological behavior of NSCLC in vivo and vitro
Bearing the analysis above in mind, we needed to further explore the effect of miR-107 on the biological behavior of A549 and H1975. We measured changes in proliferation, apoptosis and invasion of A549 and H1975 cells in transfected miR-107 mimics. Firstly, the transfection efficiency of the miR-107 mimics in A549 and H1975 was verified by RT-qPCR (Figure 2A). CCK-8 showed that overexpression of miR-107 inhibited the cell proliferation of A549 and H1975 (Figure 2B). Moreover, the flow cytometry results showed that transfection of miR-107 mimics promoted cell apoptosis of A549 and H1975 (Figure 2C). Furthermore, the results of Transwell assay showed that when miR-107 was overexpressed, the invasion of A549 and H1975 was suppressed (Figure 2D). In addition, tumor volume and weight in the miR-107 mimics group were significantly lower in comparison with the NC group in vivo (Figure 2E,F,G). Therefore, overexpression of miR-107 inhibited the malignant biological behavior of NSCLC in vivo and vitro.
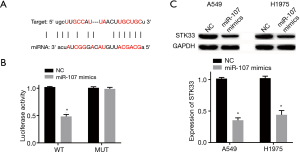
miR-107 targeting STK33
miRNAs regulate gene expression by changing the stability or translation efficiency of targeted mRNAs. The miR-107 and STK33 binding sites were discovered with bioinformatics databases (Figure 3A). The Dual-Luciferase reporter gene results showed that overexpression of miR-107 decreased the luciferase activity of STK33 WT reporter but had no effect on the STK33 MUT reporter in 293T cells (Figure 3B). Moreover, Western blotting revealed overexpression of miR-107 to have a reducing effect on the expression levels of STK33 in both A549 and H1975 (Figure 3C). Thus, it can be seen that miR-107 targeted STK33 and downregulated its expression in A549 and H1975.
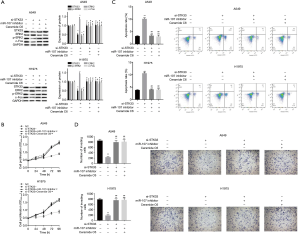
The proliferation, invasion and promoted apoptosis of NSCLC cells was inhibited by miR-107 through regulated STK33/ERK signaling pathway in vitro
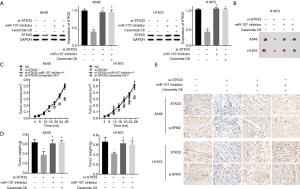
To further examine the mechanism behind the effect of miR-107 on cell proliferation, apoptosis, and invasion in NSCLC through the regulation of the STK33/ERK signaling pathway in vitro, we first measured the expression of STK33 in si-STK33, si-STK33 + miR-107 inhibitor and si-STK33 + ceramide C6 transfected A549 and H1975 cells (Figure 4A). Notably, ceramide C6 is an activator of the ERK signaling pathway. CCK-8 showed that si-STK33 significantly inhibited cell proliferation in A549 and H1975 compared with the NC, si-STK33+miR-107 inhibitor, and si-STK33 + Ceramide C6 groups (Figure 4B). In addition, the flow cytometry results showed that si-STK33 promoted cell apoptosis, but the results of the si-STK33 + miR-107 inhibitor and si-STK33 + Ceramide C6 groups showed no significant difference from the NC group (Figure 4C). Moreover, the Transwell results showed that si-STK33 suppressed cell invasion in A549 and H1975, while si-STK33 + miR-107 inhibitor and si-STK33 + Ceramide C6 enhanced cell invasion (Figure 4D). Therefore, miR-107 inhibited cell proliferation, invasion and promoted apoptosis of NSCLC by regulating the STK33/ERK signaling pathway in vitro.
miR-107 suppressed tumor growth of NSCLC via STK33/ERK signaling pathway in vivo
To verify that miR-107 impacts NSCLC tumor growth via the STK33/ERK signaling pathway in vivo, firstly, the expression level of STK33 in each group was measured by Western blotting (Figure 5A). As the results showed, both the tumor volume and weight were remarkably decreased in the si-STK33 group, but the si-STK33 + miR-107 inhibitor and si-STK33 + Ceramide C6 groups showed no significant difference to the NC group (Figure 5B,C,D). Furthermore, the immunohistochemistry staining results showed that the expressions of STK33 and p-ERK2 were lower in the si-STK33 group than in the NC, si-STK33+miR-107 inhibitor, or si-STK33 + Ceramide C6 groups (Figure 5E). Consequently, miR-107 suppressed tumor growth in NSCLC via the STK33/ERK signaling pathway in vivo.
Discussion
Due to its high levels of recurrence, metastasis, and drug resistance, the idea of targeted gene therapies for NSCLC has been put forward by recent treatment developments (13). miRNAs may play a role as oncogenes or tumor suppressor genes in cancer, thus miRNA-based therapy ushers in a new era in cancer management (14). In our study, we expounded that miR-107 acted as a tumor suppressor gene in NSCLC, inhibiting malignant biological behavior of NSCLC via regulation of the STK33/ERK signaling pathway in vivo and vitro.
Abnormal expression of miR-107 is related to many cancers. miR-107, for example, regulates tumor progression in gastric cancer (15) and acts as a tumor suppressor in ovarian cancer by blocking cell cycle (16). Moreover, miR-107 inhibits the proliferation of prostate cancer cells (17). In NSCLC, miR-107 expression has been reported to be reduced, and lower expression of miR-107 has been closely associated with NSCLC tumor progression and reduced survival rate in patients (18). Our results also reported that miR-107 was downregulated in cell lines and tissues of NSCLC patients. Furthermore, overexpression of miR-107 inhibited the malignant biological behavior of NSCLC cell lines. Beyond this, other studies have found miR-107 to be related to drug resistance in NSCLC. For example, in one study, miR-107 developed a critical role in drug resistance to cisplatin by means of targeting the CDK8 protein in NSCLC cell lines (19). Additionally, miR-107 strengthens the chemotherapy activity of paclitaxel by means of targeting apoptosis factor Bcl-w in NSCLC (20). Thus, the direction of our future research may be to explore the extent to which miR-107 regulates drug resistance in NSCLC by regulating STK33.
This study found that miR-107 influenced the malignant biological behavior of NSCLC by regulating STK33 expression, focusing on the role of STK33 in tumorigenesis, which is a new protein that has attracted much attention in research in recent years (21). It has been proved that STK33 increases the viability of cancer cells in a kinase activity-dependent way by inhibiting mitochondrial apoptosis (22). Therefore, a lot of research has proved that STK33 is involved in the development of cancer, an example of this being it behaving as a critical downstream mediator of HIF1α, promoting the growth and progression of pancreatic cancer (23). Moreover, STK33 plays crucial part in the growth and progression of pancreatic neuroendocrine tumors through its activation of the PI3K/AKT/mTOR pathway (24). Additionally, the expression levels of mRNA and STK33 were obviously decreased in lung cancer, which is related to the pathology and prognosis of lung cancer (25). More importantly, STKK33 also plays an important role in NSCLC. The potency of the STK33 gene as a therapeutic target for preventing NSCLC has previously been shown (26), and the results of this study support the above view. We found that the knockdown of STK33 results in the inhibition of cell proliferation and invasion, and the development of cell apoptosis in NSCLC.
Recently, STK33 has been proved to promote the tumorigenesis of colorectal cancer through binding with ERKs and taking part in the regulation of the ERK signaling pathway. Specifically, STK33 can phosphorylate ERK2, increasing the activity of ERK2, and activating the ERK signaling pathway to promote the tumorigenesis of colorectal cancer (11). A recent study found that the expression of p-ERK increased in NSCLC and is related to cell proliferation and migration of NSCLC (27). Furthermore, regulation of the ERK pathway through inhibited ERK phosphorylation has also been reported to lead to suppressed autophagy and augmented cell death in NSCLC (28). This study found the activation of the STK33/ERK signaling pathway led to the promotion of malignant biological behavior of NSCLC in vivo and vitro.
Conclusions
In conclusion, we found that there was a low expression of miR-107 in NSCLC tissues and cell lines. Moreover, overexpression of miR-107 inhibited malignant behavior of NSCLC and targeted STK33, downregulating its expression. The relative lack of STK33 contributed to the inhibition of cell proliferation and invasion and the promotion of cell apoptosis in NSCLC. Our study might provide a theoretical basis for NSCLC therapy.
Acknowledgments
We thank all the authors who contributed to this study.
Funding: This work was supported by Natural Science Foundation of Yunnan Province (2017FA039), Natural Science Foundation of Yunnan Province (2017FE468-214), Medical Experts Training Project of Yunnan Province (D-201641), and the National Key R&D Program of China (2017YFC0907902).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.103). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of Yunnan Cancer Hospital (NO: KY2019.57).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Naeli P, Yousefi F, Ghasemi Y, et al. The Role of MicroRNAs in Lung Cancer: Implications for Diagnosis and Therapy. Curr Mol Med 2020;20:90-101. [Crossref] [PubMed]
- Bignotti E, Todeschini P, Romani C. Identification of optimal reference RNAs to normalize miRNA expression data by qRT-PCR in formalin-fixed, paraffin-embedded lymph node tissue. Transl Cancer Res 2018;7:S733-4. [Crossref]
- Jin X, Chen Y, Chen H, et al. Evaluation of Tumor-Derived Exosomal miRNA as Potential Diagnostic Biomarkers for Early-Stage Non-Small Cell Lung Cancer Using Next-Generation Sequencing. Clin Cancer Res 2017;23:5311-9. [Crossref] [PubMed]
- Yin Z, Xu M, Li P, et al. miRNA-221 acts as an oncogenic role by directly targeting TIMP2 in non-small-cell lung carcinoma. Gene 2017;620:46-53. [Crossref] [PubMed]
- Wang YF, Ao X, Liu Y, et al. MicroRNA-608 Promotes Apoptosis in Non-Small Cell Lung Cancer Cells Treated With Doxorubicin Through the Inhibition of TFAP4. Front Genet 2019;10:809. [Crossref] [PubMed]
- Wei C, Zhang R, Cai Q, et al. MicroRNA-330-3p promotes brain metastasis and epithelial-mesenchymal transition via GRIA3 in non-small cell lung cancer. Aging (Albany NY) 2019;11:6734-61. [Crossref] [PubMed]
- Takahashi Y, Forrest AR, Maeno E, et al. MiR-107 and MiR-185 can induce cell cycle arrest in human non small cell lung cancer cell lines. PLoS One 2009;4:e6677. [Crossref] [PubMed]
- Xia H, Li Y, Lv X. MicroRNA-107 inhibits tumor growth and metastasis by targeting the BDNF-mediated PI3K/AKT pathway in human non-small lung cancer. Int J Oncol 2016;49:1325-33. [Crossref] [PubMed]
- Afonso-Grunz F, Müller S. Principles of miRNA-mRNA interactions: beyond sequence complementarity. Cell Mol Life Sci 2015;72:3127-41. [Crossref] [PubMed]
- Lindsay CR, Jamal-Hanjani M, Forster M, et al. KRAS: Reasons for optimism in lung cancer. Eur J Cancer 2018;99:20-7. [Crossref] [PubMed]
- Zhang S, Wu H, Wang K, et al. STK33/ERK2 signal pathway contribute the tumorigenesis of colorectal cancer HCT15 cells. Biosci Rep 2019;39:BSR20182351. [Crossref] [PubMed]
- Wang S, Wang SY, Du F, et al. Knockdown of PAK1 Inhibits the Proliferation and Invasion of Non-Small Cell Lung Cancer Cells Through the ERK Pathway. Appl Immunohistochem Mol Morphol 2019. [Epub ahead of print].
- Alexander M, Halmos B. VEGF inhibitors in EGFR-mutated lung cancer: a never-ending story? Ann Transl Med 2018;6:446. [Crossref] [PubMed]
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017;16:203-22. [Crossref] [PubMed]
- Wang S, Ma G, Zhu H, et al. miR-107 regulates tumor progression by targeting NF1 in gastric cancer. Sci Rep 2016;6:36531. [Crossref] [PubMed]
- Tang Z, Fang Y, Du R. MicroRNA-107 induces cell cycle arrests by directly targeting cyclin E1 in ovarian cancer. Biochem Biophys Res Commun 2019;512:331-7. [Crossref] [PubMed]
- Zhang X, Jin K, Luo JD, et al. MicroRNA-107 inhibits proliferation of prostate cancer cells by targeting cyclin E1. Neoplasma 2019;66:704-16. [Crossref] [PubMed]
- Zhong KZ, Chen WW, Hu XY, et al. Clinicopathological and prognostic significance of microRNA-107 in human non small cell lung cancer. Int J Clin Exp Pathol 2014;7:4545-51. [PubMed]
- Zhang Z, Zhang L, Yin ZY, et al. miR-107 regulates cisplatin chemosensitivity of A549 non small cell lung cancer cell line by targeting cyclin dependent kinase 8. Int J Clin Exp Pathol 2014;7:7236-41. [PubMed]
- Lu C, Xie Z, Peng Q. MiRNA-107 enhances chemosensitivity to paclitaxel by targeting antiapoptotic factor Bcl-w in non small cell lung cancer. Am J Cancer Res 2017;7:1863-73. [PubMed]
- Yin MD, Ma SP, Liu F, et al. Role of serine/threonine kinase 33 methylation in colorectal cancer and its clinical significance. Oncol Lett 2018;15:2153-60. [PubMed]
- Scholl C, Fröhling S, Dunn IF, et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell 2009;137:821-34. [Crossref] [PubMed]
- Kong F, Kong X, Du Y, et al. STK33 Promotes Growth and Progression of Pancreatic Cancer as a Critical Downstream Mediator of HIF1α. Cancer Res 2017;77:6851-62. [Crossref] [PubMed]
- Zhou B, Xiang J, Zhan C, et al. STK33 promotes the growth and progression of human pancreatic neuroendocrine tumour via activation of the PI3K/AKT/mTOR pathway. Neuroendocrinology 2020;110:307-20. [Crossref] [PubMed]
- Lu Y, Tang J, Zhang W, et al. Correlation between STK33 and the pathology and prognosis of lung cancer. Oncol Lett 2017;14:4800-4. [Crossref] [PubMed]
- Sun EL, Liu CX, Ma ZX, et al. Knockdown of human serine/threonine kinase 33 suppresses human small cell lung carcinoma by blocking RPS6/BAD signaling transduction. Neoplasma 2017;64:869-79. [Crossref] [PubMed]
- Chowdhury P, Dey P, Ghosh S, et al. Reduction of metastatic potential by inhibiting EGFR/Akt/p38/ERK signaling pathway and epithelial-mesenchymal transition after carbon ion exposure is potentiated by PARP-1 inhibition in non-small-cell lung cancer. BMC Cancer 2019;19:829-45. [Crossref] [PubMed]
- Meng J, Chang C, Chen Y, et al. EGCG overcomes gefitinib resistance by inhibiting autophagy and augmenting cell death through targeting ERK phosphorylation in NSCLC. Onco Targets Ther 2019;12:6033-43. [Crossref] [PubMed]


