Hybrid approaches in atrial fibrillation ablation: why, where and who?
Introduction
Interventional treatment for symptomatic atrial fibrillation (AF) continues to evolve. The first successful surgical approach was described in 1987 and has become known as the Cox Maze procedure (1). Over the course of several years there have been modifications in surgical treatments to focus the area of incisions/ablations, standardize the approach, and maintain efficacy with reduced complications (2,3). Catheter-based approaches using radiofrequency (RF) energy to ablate cardiac tissue to replicate the surgical procedure began in the 1990’s. Then, the key discovery of pulmonary vein triggering of AF began focusing the percutaneous, approach to this area and the development of techniques of pulmonary vein isolation (PVI) (4).
Over the last 20 years, great strides have been made in both surgical and ablation techniques as well as further attempts at understanding the underlying mechanisms of AF. The hybrid AF ablation approach represents a collaborative approach between the cardiothoracic surgeon and electrophysiologist (EP) utilizing the strengths of both techniques in order to achieve outcomes that maximize success rates and minimize procedural morbidities. But which patients are candidates for this procedure? As with any cardiac procedure, patient selection is very important.
In this review we will discuss the current hybrid AF ablation approaches and discuss which patients are appropriate for the procedure.
Why hybrid AF ablation
Currently, the majority of ablations are catheter-based. In the US from 2000 to 2010, over 93,000 catheter ablations were performed for inpatients for AF (5). Including outpatient procedures, it is likely that the total number of annual AF ablation in the United States exceeds 100,000. For patients with paroxysmal AF, success rates have consistently been above 70% (6,7). However, the success rates for patients with significant cardiac disease and long-standing persistent AF remain quite low. Various adjunctive techniques, such as linear lesions, complex fractionated atrial electrogram (CFAE) mapping, bi-atrial ablations, targeting ganglionated plexi (GP) and more recently targeting rotors, have been tried with mixed successes. Why is this? Are the targeted sites of ablation appropriate or are there limitations to the endocardial approach which makes the addition of epicardial ablations advantageous?
There are several potential advantages to a hybrid approach to AF (see Table 1). With the surgical component, there is direct visualization of the myocardium to allow direct placement of lesions, more aggressive ablation at sites which may be challenging endocardially due to risk of injuring adjacent structures such as the esophagus or phrenic nerve, direct GP ablation, and the ability to occlude/amputate the left atrial (LA) appendage which serves to debulk the myocardial tissue and potentially eliminate need for anticoagulation. The endocardial approach allows more complex mapping of the LA in AF to look for either CFAEs or rotors, the ability to ensure transmural and continuous lesions, and ablation at sites which are challenging epicardially such as near the coronary sinus and circumflex artery in the left atrium (as part of the mitral isthmus lesion) and cavotricuspid isthmus in the right atrium (RA).
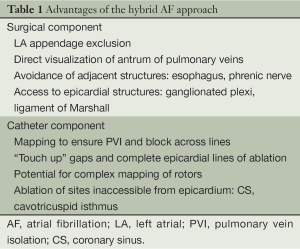
Full table
Components of hybrid AF ablation
There are several variations to the hybrid AF ablation, but the primary components are PVI with LA linear lesions, and endocardial confirmation and additional lesions to assure conduction block. Adjunctive measures include targeting GPs and the ligament of Marshall, complete LA “box lesion”, RA linear lesions, endocardial cavo-tricuspid ablation, and LA appendage occlusion (8).
A list of completed studies of hybrid AF ablation is shown below in Table 2. While all procedures were minimally invasive, the surgical approach varied considerably, with right, and bilateral thoracoscopic approaches as well as subxiphoid and laparoscopic access (9,10). Both unipolar and bipolar RF energy sources have been used with unique proprietary equipment. Early studies used bipolar RF energy and typically included LA appendage exclusion. Various other substrates have also been targeted, and nearly all patients had some additional ablation performed at the time of their surgical ablation. These additional lesions included additional LA ablation with roof, inferior, anterior lines, RA ablation including the cavotricuspid isthmus, coronary sinus, and GP ablation. The various ablation lesion sets are represented in Figure 1 below (8). Reported success rates for maintenance of sinus rhythm ranged from 36.8% to 92%. This is generally quite favorable and perhaps an improvement on catheter ablation alone in that most studies included a fairly high percentage of patients with persistent and long-standing persistent AF. Complication rates were low as well, but did include five deaths and three conversions to open sternotomy, as well as bleeding complications including tamponade, and pulmonary complications. Minor complications and residual morbidity were often not discussed.
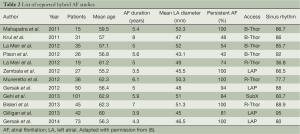
Full table
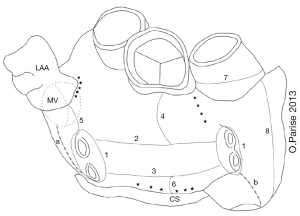
Of note, these studies differed in the timing of the endocardial mapping/ablation. Some centers performed simultaneous, convergent epicardial and endocardia ablation, while others employed a staged approach with an initial surgical procedure followed by a planned catheter procedure. Each approach has unique opportunities and challenges and each medical center seems to adapt to their own particular circumstance.
The simultaneous or convergent approach involves a laparoscopic approach crossing the diaphragm into the epicardial space via pericardial window. Linear epicardial ablation to the posterior LA is produced by unipolar RF using the Numeris® Coagulation System (nContact, Inc.) isolating the PVs along with inferior and superior lines on the posterior LA wall. These lines were completed with RF catheter ablation at the antral level of the superior left and superior and inferior right PVs. Simultaneous ablation has a significant advantage in terms of patient convenience, as only one procedure is necessary. It also allows for immediate endocardial confirmation of isolation, such that additional epicardial ablation can be performed. Theoretically this approach should eliminate concerns of tamponade or esophageal injury. However, unfortunately in early experiences complications included several mortalities from atrio-esophageal fistulae, in addition to stroke, bleeding, and pericardial effusion (11). Reported serious adverse complication rates were around 10%. However this approach can also quite time-consuming, and requires coordination of a surgical and procedural electrophysiological team. Additionally managing periprocedural anticoagulation can be challenging, and adapting the procedure space is difficult to optimize conditions for both the surgical and EP operators.
Many centers prefer a staged approach. This typically involves an initial thoracoscopic epicardial ablation followed by a catheter ablation after days to weeks. The initial epicardial ablation targets PVI as well as additional empiric substrate ablation. Over the years, the approach at the University of Virginia has evolved and currently we employ a staged-approach (Table 3). There are several important advantages to the stages approach. First, it allows optimal conditions and convenience for both the surgical and EP teams. Second, it not only allows for completion of epicardial lesions at the time of catheter ablation, but it also theoretically enables identification of areas of early reconnection which has been shown to be a major reason for failure of catheter ablation. The staged approach would also be more compatible with advanced invasive substrate mapping, including mapping and ablation of rotors which have been shown to be a major contributor to persistent AF (12).

Full table
Finally, with improvement in surgical ablation, it may be possible to employ a conditional hybrid ablation strategy, where epicardial ablation is performed with catheter ablation reserved only for patients with recurrent AF. This possibility was raised by the Brescia group; a monopolar RF system with a suction mechanism to augment tissue contact (COBRA Adhere XL™) was used to isolate the PVs en bloc with the entire posterior wall. Block was demonstrated at the time of surgical ablation with pacing from catheters inside the box lesion from the posterior epicardial surface and outside from the coronary sinus. Bidirectional block was present at the conclusion of the epicardial ablation and remained at the outset of the catheter procedure at least 30 days later in 91%; as a result in nearly 30% of patients additional endocardial ablation was not performed (13).
Concerns
Hybrid approaches to AF ablation have several important limitations. First, electroanatomic mapping cannot be performed from the epicardial surface. Hybrid approaches to ablation minimize this limitation by adjunctive endocardial mapping and ablation. However, if performed as a staged approach following epicardial ablation electroanatomic mapping is unable to guide epicardial ablation, including identifying additional triggers or substrate which may be more effectively targeted epicardially. Additionally if further substrate modification in addition to PVI is performed, including additional linear lesions, electroanatomic confirmation of block cannot be easily demonstrated at the time of surgical ablation. Ideally these linear lesions and/or substrate can be ‘touched up’ at the time of catheter ablation.
Second, with both epicardial and endocardial access patients are subjected to the risks and associated morbidity of both thoracoscopic and transvenous access including thoracic injury, pericarditis, bleeding and vascular injury, and tamponade. With staged approaches this involves additional time and costs associated with multiple procedures and convalescence. Third, the epicardial surface offers its own specific challenges. It does have additional critical structures, including the coronary arterial and venous circulation. Epicardial fat also provides a challenge, as RF energy does not easily penetrate adipose tissue. Variable atrial wall thickness and the inherent heat sink of circulating blood within the atrium also limit effectiveness of energy delivery to achieve transmural ablation lesions. Epicardial surgical approaches have addressed these difficulties by using bipolar RF energy, clamping (14), and suction adherence (13). With coronary vessels coursing in the AV groove, linear lesions cannot be anchored to fibrous base of the heart epicardially. Indeed even epicardial linear lesions performed with bipolar RF energy fail to achieve bidirectional block with gaps evident with endocardial testing in 23% of patients (15). Further endocardial ablation may be able to connect these surgical lesions; however, incomplete linear ablation in the LA has been shown to predispose patients to risks of atrial tachycardia and flutter. Managing appropriate anticoagulation is a further logistical challenge for patients with healing surgical wounds and subsequent or concurrent left-sided endocardial access. Finally, time and cost are a significant consideration especially employing a staged approach as multiple long procedures with involvement of anesthesia, surgery, and electrophysiology lab staff and operators.
Ultimately data from randomized trials comparing hybrid AF ablation to conventional catheter ablation is lacking. The fast trial randomized patients with either persistent AF or previous failed catheter ablation to repeat catheter ablation versus thoracoscopic surgical ablation alone. Surgical ablation was associated with significant improvement in efficacy at maintaining sinus rhythm at 6 months (67% vs. 44%), however surgical ablation was associated with significant and notable increases in periprocedural morbidity including pneumothorax, hemothorax, rib fracture and conversion to sternotomy in addition to embolism and tamponade with a complication rate of 23% for surgical ablation versus 9% for catheter ablation (16). There is certainly reporting bias among published studies, as several large studies of hybrid AF ablation, including the staged deep study, FAST-II, and SCALA-success have not yet been published.
Conclusions
Given that there are no published studies to date comparing hybrid ablation to catheter ablation, definitive conclusions about hybrid AF ablation cannot be made. Several different methods have been shown to be effective, however the relative efficacy compared to catheter ablation or surgical ablation alone has not been sufficiently studied for paroxysmal, persistent, or long-standing persistent AF. However, considering the advantages and disadvantages we feel that currently there are several clinical situations in which a hybrid approach should be considered; first, as adjunctive therapy for patients with symptomatic AF undergoing cardiac surgery. In this setting epicardial ablation adds little additional operative risk and can offer provide optimal access for PVI and additional anatomically guided substrate ablation as indicated. Indeed, animal studies have consistently shown the greatest degree of transmurality for epicardial RF ablation with cardioplegia. Second, hybrid ablation should be considered in patients with persistent AF who have failed catheter ablation, particularly those in whom endocardial PVI has failed because of risk to surrounding structures including phrenic nerve or esophageal injury. Hybrid approaches also would offer potential advantages for additional substrate modification including possibly posterior wall debulking, homogenization of fibrosed/scarred atrium, and atrial denervation. Efforts to elucidate non-invasive and invasive targets are ongoing and will require further study. Finally, hybrid ablation may be considered in patients with persistent and long-standing persistent AF who are not optimal candidates for catheter ablation or who prefer a thoracoscopic approach. Such situations include patients with advanced LA structural remodeling such as in valvular AF, patients following atrial septal defect closure, or patients with challenging venous access.
In conclusion hybrid epicardial and endocardial ablation for AF is promising. Further careful study of techniques and patient selection will be necessary to optimize outcomes, especially a direct comparison to catheter ablation alone.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cox JL, Schuessler RB, D'Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83. [PubMed]
- Damiano RJ Jr, Gaynor SL, Bailey M, et al. The long-term outcome of patients with coronary disease and atrial fibrillation undergoing the Cox maze procedure. J Thorac Cardiovasc Surg 2003;126:2016-21. [PubMed]
- Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126:1822-8. [PubMed]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [PubMed]
- Deshmukh A, Patel NJ, Pant S, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation 2013;128:2104-12. [PubMed]
- Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713-23. [PubMed]
- Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333-40. [PubMed]
- Gelsomino S, Van Breugel HN, Pison L, et al. Hybrid thoracoscopic and transvenous catheter ablation of atrial fibrillation. Eur J Cardiothorac Surg 2014;45:401-7. [PubMed]
- Gehi AK, Mounsey JP, Pursell I, et al. Hybrid epicardial-endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm 2013;10:22-8. [PubMed]
- Zembala M, Filipiak K, Kowalski O, et al. Minimally invasive hybrid ablation procedure for the treatment of persistent atrial fibrillation: one year results. Kardiol Pol 2012;70:819-28. [PubMed]
- Gersak B, Pernat A, Robic B, et al. Low rate of atrial fibrillation recurrence verified by implantable loop recorder monitoring following a convergent epicardial and endocardial ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:1059-66. [PubMed]
- Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628-36. [PubMed]
- Muneretto C, Bisleri G, Bontempi L, et al. Durable staged hybrid ablation with thoracoscopic and percutaneous approach for treatment of long-standing atrial fibrillation: a 30-month assessment with continuous monitoring. J Thorac Cardiovasc Surg 2012;144:1460-5. [PubMed]
- Mahapatra S, LaPar DJ, Kamath S, et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg 2011;91:1890-8. [PubMed]
- Pison L, Dagres N, Lewalter T, et al. Surgical and hybrid atrial fibrillation ablation procedures. Europace 2012;14:939-41. [PubMed]
- Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23-30. [PubMed]


