
Endobronchial ultrasound-guided injection of NBTXR3 radio-enhancing nanoparticles into mediastinal and hilar lymph nodes: a swine model to evaluate feasibility, injection technique, safety, nanoparticle retention and dispersion
Introduction
Lung cancer remains the leading cause of cancer-related death for men and women, with most patients being diagnosed at advanced stages (1). Despite the advent of molecular targeted therapies and immunotherapy, prognosis remains poor (2). Loco-regionally advanced lung cancer (stage III) is typically treated with a combination of chemotherapy, immunotherapy, and radiation therapy, but overall survival and local control are still far from ideal (3). Beyond the broadly cytotoxic effect of radiation therapy, radiation therapy can potentially boost the anti-tumor immune response to cancer through a variety of mechanisms (4-7). Hence our interest in enhancing the therapeutic effect of radiation therapy in terms of tumor destruction and modulation of the immune landscape through nanomedicine. NBTXR3 is a sterile aqueous suspension of inert hafnium oxide nanoparticles designed to generate secondary electrons and free radicals after activation by radiation, resulting in increased cell death and immune response (8). NBTXR3 has been previously studied in humans with advanced soft tissue sarcoma and showed no increased incidence of adverse events or augmentation of radiation responses in normal tissues (9). Since treatment of loco-regionally advanced lung cancer involves radiation of hilar and/or mediastinal lymph nodes (LN), in addition to injecting primary tumors, intranodal injection of NBTXR3 via endobronchial ultrasound (EBUS) may be of great use to optimize tumor control. EBUS injection of chemotherapy into peribronchial tumors and LN has been reported, but since the injected drugs are not radio-opaque, their localization within the target and surrounding tissue is unclear and there is concern for potential extravasation (10-12). The fact that the NBTXR3 is radio-opaque makes it an ideal candidate to study its distribution after EBUS LN injection. Extravasation of NBTXR3 in the mediastinum can lead to radiosensitization of adjacent structures such as large vessels, esophagus, tracheal wall, and could potentially lead to serious complications. Hence, before proceeding with human trials, we conducted a study to determine the feasibility and safety of EBUS-guided injection of NBTXR3 into swine mediastinal and hilar LN. Additionally, this study evaluated NBTXR3 retention and dispersion for up to 8 days post injection.
Methods
The University of Texas MD Anderson Cancer Center is an AAALAC-accredited institution. The Center’s institutional animal care and use committee (IACUC) approved all experimental manipulations. Swine were housed and cared for in accordance with the “Guide for the Care and Use of Laboratory Animals”, Public Health Service policy, and the Animal Welfare Act and Animal Welfare Regulations.
NBTXR3 is a sterile aqueous suspension of functionalized hafnium oxide nanoparticles with a negative surface charge at neutral pH. The electrostatic repulsions between the charged nanoparticles, ensures the stability of the NBTXR3 suspension. The size of the nanoparticles, measured by dynamic light scattering (DLS), is centered at 50 nm (13).
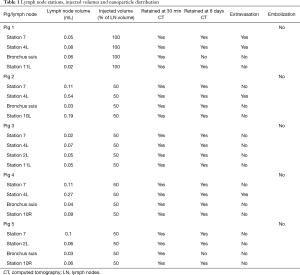
Five domestic pigs were utilized for this study. All animals underwent the following procedures. On day 1, pigs were placed under general anesthesia using Telazol (4.4–6.6 mg/kg IM), Propofol (4 to 12 mg/kg/h), Meloxicam (0.4 mg/kg) and Isoflurane to effect, receiving mechanical ventilation through an endotracheal tube. Standard vital signs monitoring was recorded. All computed tomography (CT) imaging was performed on a 128-slice CT scanner (Somatom Definition Edge, Siemens Healthineers, Germany) using standard chest CT protocols. A baseline chest CT with intravenous (IV) contrast (iodine-based) was performed to assess for mediastinal and hilar LN. At least 2 mediastinal and 2 hilar LN were selected, and their volume was calculated multiplying their 3 dimensions on chest CT. An EBUS exam was performed (BF-UC180F, Olympus America) to ensure these LN could be localized. Within 30 min of the first CT, a second chest CT, now without contrast, was performed to assure the IV contrast had been completely “washed out”. This step was performed because NBTXR3 is radio-opaque and looks similar to IV contrast on CT. Once this second chest CT corroborated that the iodine contrast was absent, we proceeded with EBUS injection of NBTXR3 into the target LN. Injections were made with a dedicated 22G needle (Olympus America) under real-time EBUS image guidance and simultaneous fluoroscopy. The injected NBTXR3 volume was based on the volume calculated for each LN by CT as described above. Though the originally planned injection volume was 25% of calculated LN volume, we encountered extremely small LN which would make it difficult to visualize NBTXR3 during the injection process using fluoroscopy. A decision to inject 50% and 100% of the calculated LN volume was made to determine which of those volumes allowed NBTXR3 visualization with fluoroscopy during injection, while also hypothetically avoiding a significant increase of intra-nodal pressure that could result in capsule disruption and nanoparticle extravasation. For the first animal, 100% of the estimated LN volume (of each LN) was injected, with 2 intranodal injections (25% for the first one and 75% for the second one). All remaining animals (4) then received 50% of LN volume, administered in one injection, which was easily seen by fluoroscopy and CT. At the 5- and 30-minute post LN injection time point, animals underwent a third and fourth chest CT, both of these without contrast, to evaluate NBTXR3 retention and dispersion. Additionally, on day 1, blood was drawn at baseline (pre-injection) and 30 min post-injection to survey for acute reactant factors and blood counts. Pigs were then extubated, recovered, housed and monitored (behavior and vital signs). On day 8, pigs received a fifth CT scan under anesthesia including both chest and abdomen, to study NBTXR3 retention, dispersion and to evaluate for signs of embolized particles. Blood was drawn and the animals were subsequently euthanized and necropsied. During necropsy, mediastinal and hilar LN were dissected for gross and histologic evaluation. Both injected LN and non-injected LN (control) were dissected and analyzed.
Nanoparticles retention and dispersion was evaluated by comparing all 3 post-injection chest CT scans (5 min, 30 min, and 8 days after injection). Retention was defined as presence of greater than 80% of the “original” NBTXR3 radio-opaque volume (“original” based on CT findings at 5 min post-injection scan), and assessed both at 30 minutes and 8 days CT scans. Dispersion was described based on CT findings and defined as absence or presence of nanoparticles immediately outside the LN (extravasation) or in distant organs (nanoparticle embolization). The unit of analysis was the injected LN for retention and extravasation, and the animal for nanoparticle embolization.
Results
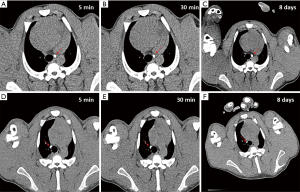
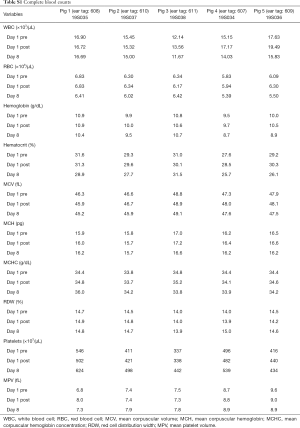
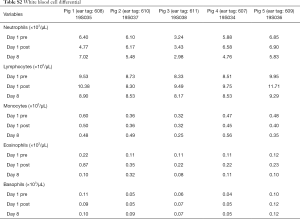
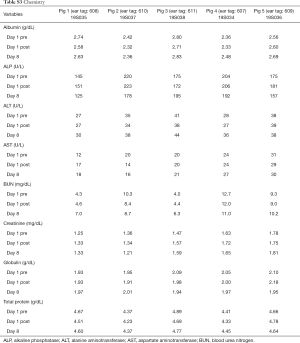
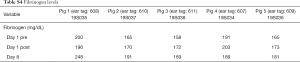
A total of 5 pigs, 2 females, weighting between 47 and 53.4 kg were included in the study. Twenty LN (4 LN per animal) were injected with NBTXR3. A description of LN stations, estimated LN volume, injected volume, and nanoparticle distribution can be found in Table 1. NBTXR3 was retained in 100% of LN at 30 min, and 90% of LN at 8 days (see Figure 1). There was minor NBTXR3 extravasation (outside of the LN) in 4 out of the 20 injections. There were no cases of embolization visible by CT in distant organs. In 3 of the 20 injections, small air-bubbles were introduced in the targets and surrounding tissue along with the NBTXR3 injection. Of note, at 8 days, none of these air-bubbles were present on repeat CT scan (see Figure 2). The time required for bronchoscopy in each animal—including the loading and injecting of the exact volume of NBTXR3 in 4 different LN—was 52, 15, 16, 10, and 14 minutes for animals 1, 2, 3, 4, and 5, respectively. Laboratory findings revealed stable white blood cell counts, platelet counts, kidney function, liver enzymes, albumin, globulin and fibrinogen at days 1 and 8 post LN injections (see Tables S1-S4). These laboratory values did not show any evidence of acute inflammation with NBTXR3 injection. There were no intra-procedural complications and no adverse events in any of the pigs during the follow-up period of 8 days. Chest CT scan done at day 8 did not show any complications either.
Full table
Full table
Full table
Full table
Full table
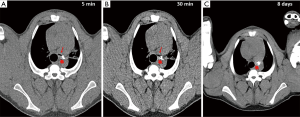
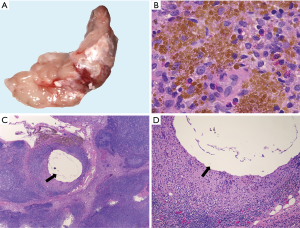
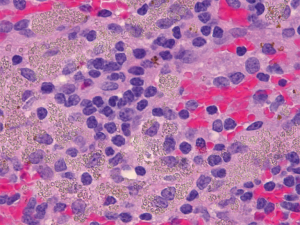
Histologic features common to both injected and control LN included marked sinus histiocytosis, abundant large granular lymphocytes, and mildly increased numbers of eosinophils and neutrophils. Injected LN showed the expected evidence of trauma. There was evidence of local hemorrhage, such as macrophages containing intracytoplasmic golden-brown material (hemosiderin), and focal defects surrounded by epithelioid macrophages and Langhans-type multinucleated giant cells, both the result of needle insertion (see Figure 3). Eight out of 14 injected and 4 out of 13 non-injected LN showed hemosiderin-laden macrophages. Multinucleated giant cells were observed in 2/14 injected and 2/13 non-injected LN. The finding of hemorrhage, hemosiderin-laden macrophages, and multinucleated giant cells in some non-injected LN is not surprising considering the interconnectedness of the lymphatic system. We also encountered macrophages with tan-gray refractive material—probably NBTXR3—as shown in Figure 4 in injected LN.
Discussion
This animal study corroborates that EBUS-guided injection of NBTXR3 in both hilar and mediastinal LN can be performed safely with a high retention rate, low extravasation rate, and no CT-visible distant nanoparticle embolization. The radio-opacity of these nanoparticles allowed accurate study of their distribution with CT scan. The high retention rate at 8 days is of relevance since it provides information regarding the time window between nanoparticle injection and future radiation and assurance that therapeutic radio-enhancement will occur where NBTXR3 was originally injected. No bronchoscopic complications, loco-regional or systemic inflammatory response were seen with NBTXR3 injection.
To the best of our knowledge, this is the first report of EBUS-guided injection of a radiopaque aqueous substance into mediastinal and hilar LN. With our bronchoscopy field moving towards therapeutics and with the growing interest in tumor or LN injection with chemotherapeutic agents, we believe our study provides highly valuable technical information to bronchoscopists.
Several challenges were encountered during this study. The first one, and probably the main limitation, being the unusually small LN volumes. Given the extremely small LN volumes encountered in the pigs of this study, a NBTXR3 injection volume equivalent to the 25% of the LN volume was not easily visualized by fluoroscopy during injection. Hence, product volumes equivalent to 50% and 100% of LN volume were also utilized. For the purpose of this study, 50% of LN volume was enough NBTXR3 to be visualized during injection. Nevertheless, finding the appropriate injection volume was not the goal of this study. Since LN involved by malignancy in humans are much larger than the ones we injected in our pigs, we do not foresee this to be a problem.
Another encountered challenge was the introduction of air into the LN or mediastinum that occurred with some of the injections. The amount of air was small, and it should not be greater than the volume of the lumen of the utilized injection needles (0.3 mL). Although pre-filling the needles with the NBTXR3 to evacuate the air inside may seem like straightforward solution, preventing spillage of NBTXR3 in the airways when passing the needle through the bronchoscope was also needed. Therefore, after pre-filling the needle lumen with NBTXR3 solution, we inserted the tip of the needle into sterile bone wax to create a plug and avoid spillage of NBTXR3 in the airways. This technique was successful in preventing introduction of air into LN without causing spillage in the airways. While carrying out this experiment, the significance of a few air-bubbles being introduced into LN targets or in the mediastinum was unknown, thus a technique was developed to prevent it. Of note, the repeat chest CT at 8 days showed that these small air bubbles had completely disappeared, and hence they may not pose a concern in future human studies.
Extravasation outside the LN in 4 out of our 20 injections was experienced. Since LN are encapsulated organs, the combination of capsule perforation by the needle and the increased intra-nodal pressure as a result of the added volume could explain this phenomenon. Extravasation will likely be less often in future human trials where larger LN will only be injected with 25% of their volume. Nevertheless, based on the follow up chest CTs and necropsy findings, this extravasation was not associated with any significant local or systemic inflammatory reaction. Distal embolization of NBTXR3 was not evidenced by chest/abdomen/pelvis CT. However, embolization to the brain or embolization of very small amounts of nanoparticles that may not be detected by CT technology cannot be ruled out in this study. Since the original injected volume is retained, as observed on the eighth day CT scan, any distal nanoparticle embolization in our study was likely negligible. Nanobiotix has conducted hafnium quantification in serum before by inductively coupled plasma mass spectrometry (ICP-MS). Hafnium quantification is performed in all clinical studies after intratumoral injection of NBTXR3. Across studies, negligible concentrations of hafnium, quantified by ICP-MS, were present in blood (8).
The safety and feasibility of EBUS-guided NBTXR3 injection in swine mediastinal and hilar LN has been confirmed. No complications during bronchoscopy, and no local or systemic inflammatory reactions to NBTXR3 injection were seen. Animals were observed for 8 days after injection with behavioral and vital sign documentation, showing no abnormalities. Although the injected suspension is sterile, the needles utilized for injections can potentially become contaminated with bacteria when passed through the working channel of the bronchoscope. Henceforth, one of our concerns was the possibility of causing infectious mediastinitis. Fortunately, analysis of complete blood count and serum acute phase reactants failed to show any indication of acute inflammation. Of note, malignant LN in humans may have abundant necrosis and liquefied material inside, increasing the risk for infection, and hence this potentially serious complication should still be kept in mind when performing human trials. CTs at day 8 did not show pneumothorax, pneumomediastinum, mediastinitis, or large distant embolization. Gross pathology and histopathologic analysis post-necropsy confirmed the above findings. These findings provide a safety signal for this technique. Larger animal studies and human trials need to be conducted to corroborate these findings.
Conclusions
EBUS-guided injection of NBTXR3 radio-enhancing nanoparticles can be performed achieving a high rate of nanoparticle retention, low extravasation, and no significant nanoparticle embolization. Retention at day 8 allows for an ample window for radiation sensitization post injection. Human trials to document its safety are needed.
Acknowledgments
We sincerely thank Jennifer Meyer, Robin Harmon, and Stephanie Mayor for providing anesthesia and overall animal care, Taylor Lee Jr. for his assistance with bronchoscopy, and Keith A. Michel for his assistance in image processing. Our study would have not been possible without their help. We would also like to thank the John S Dunn Research Foundation Center for Radiological Sciences for providing imaging and lab support.
Funding: This study was sponsored by Nanobiotix and in part by the National Institute of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.100). RFC reports grants from Nanobiotix, during the conduct of the study; grants from Concordia, grants from Siemens, grants and personal fees from Olympus, outside the submitted work and serves as an unpaid editorial board member of Journal of Thoracic Disease from Apr 2020 to Mar 2022. RRA is currently an employee of Nanobiotix. SL reports grants from Nanobiotix, during the conduct of the study. SFS reports grants from Varian, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional animal care and use committee (IACUC) of The University of Texas MD Anderson Cancer Center (No. 0001933).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts and Figures 2018.
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Rajappa S, Sharma S, Prasad K, et al. Unmet Clinical Need in the Management of Locally Advanced Unresectable Lung Cancer: Treatment Strategies to Improve Patient Outcomes. Adv Ther 2019;36:563-78. [Crossref] [PubMed]
- Filippi AR, Di Muzio J, Badellino S, et al. Locally-advanced non-small cell lung cancer: shall immunotherapy be a new chance? J Thorac Dis 2018;10:S1461-7. [Crossref] [PubMed]
- Skrzypski M, Jassem J. Consolidation systemic treatment after radiochemotherapy for unresectable stage III non-small cell lung cancer. Cancer Treat Rev 2018;66:114-21. [Crossref] [PubMed]
- Tabchi S, Kassouf E, Rassy EE, et al. Management of stage III non-small cell lung cancer. Semin Oncol 2017;44:163-77. [Crossref] [PubMed]
- Kumar SS, Higgins KA, McGarry RC. Emerging Therapies for Stage III Non-Small Cell Lung Cancer: Stereotactic Body Radiation Therapy and Immunotherapy. Front Oncol 2017;7:197. [Crossref] [PubMed]
- Bonvalot S, Le Pechoux C, De Baere T, et al. First-in-Human Study Testing a New Radioenhancer Using Nanoparticles (NBTXR3) Activated by Radiation Therapy in Patients with Locally Advanced Soft Tissue Sarcomas. Clin Cancer Res 2017;23:908-17. [Crossref] [PubMed]
- Bonvalot S, Rutkowski PL, Thariat J, et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2-3, randomised, controlled trial. Lancet Oncol 2019;20:1148-59. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Darwiche K, et al. Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des Devel Ther 2013;7:571-83. [PubMed]
- Khan F, Anker CJ, Garrison G, et al. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann Am Thorac Soc 2015;12:101-4. [Crossref] [PubMed]
- Mehta HJ, Begnaud A, Penley AM, et al. Treatment of isolated mediastinal and hilar recurrence of lung cancer with bronchoscopic endobronchial ultrasound guided intratumoral injection of chemotherapy with cisplatin. Lung Cancer 2015;90:542-7. [Crossref] [PubMed]
- Marill J, Anesary NM, Zhang P, et al. Hafnium oxide nanoparticles: toward an in vitro predictive biological effect? Radiat Oncol 2014;9:150. [Crossref] [PubMed]


