
Lung cancer and obstructive lung disease in never smokers
Introduction
In surgical lung cancer therapy, to evaluate the details of another lung disease is one of the essential factors to accurate treatment policy. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define chronic obstructive pulmonary disease (COPD) is a disorder characterized by persistent respiratory symptoms and airflow restriction. The World Health Organization predicts that COPD will be the third leading cause of death in the world by 2030 (1); moreover, 3 million people died of COPD in 2016 (2).
COPD is a disease that can be caused by smoking; however, the number of patients with COPD who have never smoked still exists worldwide. Approximately 30% of patients with COPD from each community have never smoked (3). The differences between smoking-induced COPD and COPD unrelated to smoking are still unknown.
In worldwide, lung cancer has a high mortality rate and rank within the top 10 causes of death (2). COPD is often found as a comorbidity in patients with lung cancer. Nearly 10% of patients with COPD develop lung cancer (4). Moreover, almost 50% of patients with lung cancer have COPD (5). The reason possibly includes the mechanism of oncogenesis and the onset of obstructing disease as a commonality (6). It is well-known that COPD is an independent prognostic factor of lung cancer survival.
Akamine et al. focused on patients who had never smoked. They reported the patients with obstructive lung disease (OLD) who were operated for lung cancer in their single institution and revealed OLD was an independent negative prognostic factor of lung cancer survival in never smokers (7).
They retrospectively examined 244 never-smokers and 304 smokers with surgically resected non-small cell lung cancer (NSCLC) from January 2003 to December 2014. Other causes of death or death within 30 days after surgery were eliminated. OLD is defined based on the preoperative spirometry test score of the forced expiratory volume in 1 second (FEV1)/forced volume vital capacity (FVC) ratio is less than 70%.
Cancer-specific survival (CSS), age, pathological status, vascular invasion, and OLD were independent predictive factors of never smoker patients. In recurrence-free survival, there is no association with CSS and OLD patients with smoking history. In overall survival, OLD was a predictive risk factor, particularly in never smokers.
They emphasized their report is important because they focused on never smoked patients with NSCLC patients are increasing, and the new facts are revealed firstly.
The possibility of the original mechanisms of lung cancer with OLD in never smokers was discussed, and the PI3KCA gene is possibility to be a distinctive feature of NSCLC patients with OLD even in case of never smokers, although it remains to be investigating. As another factor, low socioeconomic status can be effect imbalance in lung cancer survival. Because despite the adjustment of prognosis for gender or age, OLD still reveled poor prognosis.
One of the limitations of the study is including untreated with bronchodilator OLD patients. Not only COPD, but also Asthma, and so on are including in the OLD. And second-hand smoke exposure was difficult to evaluate from the history.
Regarding the primary finding by Akamine et al., we aimed to investigate the connection between OLD disease represented by COPD and lung cancer in never smoker.
Risk factors of COPD and prognostic factors of COPD with lung cancer
The major risk factors of COPD can be internal or external.
First-hand smoke is the most important external risk factor (8). Second-hand smoke (9), air pollution (10), exposure to occupational dust and chemicals (11), and biomass burning flame (12) are also major external risk factors. Patients of passive smoking are possibly included in never smoker groups. A total of 21,400 people died of lung cancer and 603,000 deaths were attributable to second-hand smoke worldwide (13).
Childhood or adulthood respiratory infections (14), smoking during pregnancy (15), history of pulmonary tuberculosis (16), and socioeconomic factors (17) are also external risk factors. While, the internal risk factors include genetic mutations (18), airway sensitivity (19), familial history of COPD (20), autoimmunity (21), and aging (22). It is difficult to evaluate each risk factor alone, and some risk factors may simultaneously affect patients. These risk factors make people more susceptible to chronic disease. The deficiency of α-antitrypsin is an established genetic risk factor. Other candidate genetic risk factors are etoposide hydrolase, glutathione S-transferase, heme oxygenase-1, matrix metalloproteinase (23,24), and inflammation-related genes (24).
The oncogenesis of lung cancer and the onset of obstructing lung disease have a mutual mechanism, and smoking is a major and relatively well-evaluated common risk factor (25).
Oxidative stress (26), chronic inflammation (27), genetic factors (28), the involvement of proteolytic enzymes, aging, and epigenetic factors such as telomere shortening, DNA methylation, and histone modification are believed to be related (26). It is believed that smoking causes oxidative stress, chronic inflammation, DNA damage, and mismatch repair. In lung cancer with COPD unrelated to tobacco, some of these mechanisms are suggested to be involved in oncogenesis.
The prognosis of COPD with lung cancer in never smokers will have some common factors in the case of smokers. To explain the correlation mechanism between COPD in smokers and lung cancer, a genome-wide association study revealed susceptibility genome types overlap, such as CHRNA3/5, which concerns nicotine dependence in COPD and lung cancer (29,30). In a meta-analysis study by Jemma et al. (31), CHRNA3/5 gene variants mediate airflow obstruction regardless of smoking history. COPD is a clinically and pathologically heterogeneous disease (32). There are multiple conditions of the disease and prognosis, which are divided into clinical phenotypes. The level of airflow limitation, symptoms, and frequency of exacerbations is a clinical measurement for treatment and prognostic factors. Factors such as age (33), smoking index, FEV1 (33), the emphysematous lesion (34), hypoxemia (35), pulmonary hypertension (36), and systemic complications (37) including lung cancer can also affect the prognosis. Analysis of the pathological condition and its differences in each patient along with the genotype and endotype is ongoing worldwide (38). COPD is not only an oncogenic risk factor, but it also affects the overall and disease-free survival in patients with lung cancer (24). In cases where the patient has undergone surgery, the presence of severe COPD classified as GOLD 2/3 is a prognostic risk factor and can affect disease-free survival in patients with early lung cancer (39). Even in stage IA of NSCLC, Yoshida et al. reported that in patients classified as GOLD 2/3-COPD, 46.0% had postoperative pulmonary complications that led to poor prognosis (40). Carr et al. reported that visual emphysema, airflow obstruction as measured by FEV1.0, is an independent predictive risk factor of lung cancer in the current smoker and never smoker patients (41). They suggest that acute respiratory exacerbations should also be considered when screening patients because of the increased risk of lung cancer and small cell lung cancer than NSCLC.
COPD is not only an oncogenic risk factor, but it also affects the overall and disease-free survival in patients with lung cancer (24). In cases where the patient has undergone surgery, the presence of severe COPD classified as GOLD 2/3 is a prognostic risk factor and can affect disease-free survival in patients with early lung cancer (39). Even in stage IA of NSCLC, Yoshida et al. reported that in patients classified as GOLD 2/3-COPD, 46.0% had postoperative pulmonary complications that led to poor prognosis (40). Carr et al. reported that visual emphysema, airflow obstruction as measured by FEV1.0, is an independent predictive risk factor of lung cancer in the current smoker and never smoker patients (41). They suggest that acute respiratory exacerbations should also be considered when screening patients because of the increased risk of lung cancer and small cell lung cancer than NSCLC. Prognostic factors of COPD with lung cancer in never smoker are controversial, the decline of FEV1 and the emphysematous lesion will be a candidate (Figure 1).
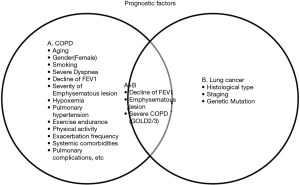
Akamine et al. focused on the possibility of the PIK3CA driver gene mutation as a potential pathogenic mechanism in the development of OLD in patients who had never smoked and had lung cancer (7). This was based on a previous study by Sawa et al., which insists that the PIK3CA mutation is a characteristic genetic feature of NSCLC with COPD, regardless of age, amount of smoking, pathological stage, and histology (42). No similar literature focused on the PI3KCA mutation in COPD-based lung cancer.
Phosphoinositide 3-kinase (PI3K) is a lipid kinase that regulates cell growth or apoptosis. Nicotine enhances cell migration through the PI3K pathway and yields to lung cancer cell invasion and metastasis (43). PI3K is classified into 4 classes: IA, IB, II, and III. The PI3CA gene is coded with a PI3K with a p110α unit, which is situated in class IA. PIK3CA mutant expression is well known in solid cancers and it activates lipid kinase and PI3K/AKT signaling (44). In NSCLC, the prevalence is approximate 2–4% and is higher in squamous cell carcinoma (45). In adenocarcinoma and squamous cell carcinoma, PIK3CA sometimes has mutations that coexist with other mutations of oncogenes such as EGFR, KRAS, BRAF, and ALK (45-47).
PIK3CA mutation also detects other colon cancers: breast, liver, and urinary bladder (48). It is not only present in malignant disease but also the overgrowth of body parts in the embryonic development, defined as PIK3CA-related overgrowth spectrum (PROS). PIK3CA mutation and PROS clusters concentrate on correlated hotspots, E542, E545, and H1047 (48).
PI3KCA mutation may be a genetic factor of NSCLC in never smokers; however, this is difficult to evaluate because it functions similarly to the nicotine contained in tobacco as a point of PI3K/AKT pathway activation, causing cancer progression.
Prognosis of lung cancer coexisting with OLD
A FEV1.0/forced vital capacity (FVC) ratio <bvvc0.7 is the common standard of OLD, which includes asthma, bronchiolitis, bronchiectasis, and the combination of chronic bronchitis and emphysema-related diseases.
In COPD, the clinical condition boundaries are unclear. Approximately 15% of COPD symptoms overlap with asthma (49), which is called the asthma-COPD overlap syndrome (ACOS). Approximately 6% of COPD symptoms coexist with fibrosis (4), which is called the combined pulmonary fibrosis and emphysema (CPFE).
In asthma and ACOS, the correlation between the carcinogenesis of lung cancer is unclear. Harada et al. insist that asthma and ACOS patients frequently end up dying because of malignant disease (50).
In 2005, Cottin et al. advocated that CPFE should be defined as emphysema in the upper lung field and fibrosis in the lower lung field as observed in chest computed tomography. A total of 50% of patients with CPFE have lung cancer (51), which is more frequent than COPD or fibrosis alone (52). The typical histological type of lung cancer with COPD (53) or CPFE is squamous cell carcinoma. However, the prognosis is worse for patients with CPFE, especially in patients who have undergone surgery (52). The difference between high carcinogenicity and high mortality rate means indicates that image evaluation will be considered in the study of the connection between OLD and lung cancer.
In the study of Akamine et al., OLD was defined as FEV1/FVC <70% and possibly included asthma or other obstructive diseases. Furthermore, normal respiratory function with emphysematous changes in the images is excluded, although nonemphysematous COPD is included. In the evaluation of nonsmoker lung cancer patients, a detailed medical history, respiratory function evaluation, and image evaluation are essential.
Conclusions
COPD is known as an independent risk factor for lung cancer in smokers. Akamine et al. reported OLD is as well in the never smoked lung cancer patients. OLD broadly defines the obstructive disease, it is controversial whether each disease or condition including in OLD needs to be examined or not. The multidimensional aspects of the COPD may need evaluation because of the clinical phenotypes and overlapping peripheral diseases such as CPFE that affect prognosis.
In never smokers, risk factors for oncogenesis are difficult to evaluate because they are more diverse than those in smokers. PI3KCA mutation may be one of the genetic characteristics, considering PI3K/AKT pathway activation because of solid cancer progression in other organs or PROS. Further observation and investigational studies are required.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.29). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. COPD predicted to be third leading cause of death in 2030. 2008. Available online: https://www.who.int/respiratory/copd/World_Health_Statistics_2008/en/. Accessed 03.15 2020.
- WHO. The top 10 causes of death. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 03.15 2020.
- Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax 2015;70:822-9. [Crossref] [PubMed]
- Divo M, Cote C, De Torres JP, et al. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2012;186:155-61. [Crossref] [PubMed]
- Loganathan RS, Stover DE, Shi W, et al. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest 2006;129:1305-12. [Crossref] [PubMed]
- Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer 2013;13:233-45. [Crossref] [PubMed]
- Akamine T, Tagawa T, Shimokawa M, et al. The prognostic impact of obstructive lung disease on survival of never smokers with resected non-small-cell lung cancer: a comparison with smokers. Interact Cardiovasc Thorac Surg 2019;28:735-43. [Crossref] [PubMed]
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-55. [Crossref] [PubMed]
- Eisner MD. Secondhand Smoke and Obstructive Lung Disease. Am J Respir Crit Care Med 2009;179:973-4. [Crossref] [PubMed]
- Kurmi OP, Semple S, Simkhada P, et al. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax 2010;65:221-8. [Crossref] [PubMed]
- Boschetto P, Quintavalle S, Miotto D, et al. Chronic obstructive pulmonary disease (COPD) and occupational exposures. J Occup Med Toxicol 2006;1:11. [Crossref] [PubMed]
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology 2016;21:14-23. [Crossref] [PubMed]
- Oberg M, Jaakkola MS, Woodward A, et al. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 2011;377:139-46. [Crossref] [PubMed]
- Sethi S. Bacterial Infection and the Pathogenesis of COPD. Chest 2000;117:286S-91S. [Crossref] [PubMed]
- Beyer D, Mitfessel H, Gillissen A. Maternal smoking promotes chronic obstructive lung disease in the offspring as adults. Eur J Med Res 2009;14 Suppl 4:27-31. [Crossref] [PubMed]
- Caballero A, Torres-Duque CA, Jaramillo C, et al. Prevalence of COPD in Five Colombian Cities Situated at Low, Medium, and High Altitude (PREPOCOL Study). Chest 2008;133:343-9. [Crossref] [PubMed]
- Prescott E, Vestbo J. Socioeconomic status and chronic obstructive pulmonary disease. Thorax 1999;54:737-41. [Crossref] [PubMed]
- Shapiro SD. Animal Models for COPD. Chest 2000;117:223S-7S. [Crossref] [PubMed]
- Scichilone N, Battaglia S, La Sala A, et al. Clinical implications of airway hyper-responsiveness in COPD. Int J Chron Obstruct Pulmon Dis 2006;1:49-60. [Crossref] [PubMed]
- Hersh CP, Hokanson JE, Lynch DA, et al. Family History Is a Risk Factor for COPD. Chest 2011;140:343-50. [Crossref] [PubMed]
- Kheradmand F, Shan M, Xu C, et al. Autoimmunity in chronic obstructive pulmonary disease: clinical and experimental evidence. Expert Rev Clin Immunol 2012;8:285-92. [Crossref] [PubMed]
- Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest 2009;135:173-80. [Crossref] [PubMed]
- DeMeo DL, Hersh CP, Hoffman EA, et al. Genetic determinants of emphysema distribution in the national emphysema treatment trial. Am J Respir Crit Care Med 2007;176:42-8. [Crossref] [PubMed]
- Hersh CP, Demeo DL, Lange C, et al. Attempted replication of reported chronic obstructive pulmonary disease candidate gene associations. Am J Respir Cell Mol Biol 2005;33:71-8. [Crossref] [PubMed]
- Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology 2016;21:269-79. [Crossref] [PubMed]
- Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer 2015;90:121-7. [Crossref] [PubMed]
- Brody JS, Spira A. State of the Art. Chronic Obstructive Pulmonary Disease, Inflammation, and Lung Cancer. Proc Am Thorac Soc 2006;3:535-7. [Crossref] [PubMed]
- Young RP, Hopkins RJ. How the genetics of lung cancer may overlap with COPD. Respirology 2011;16:1047-55. [Crossref] [PubMed]
- Caporaso N, Gu F, Chatterjee N, et al. Genome-Wide and Candidate Gene Association Study of Cigarette Smoking Behaviors. PLoS One 2009;4:e4653. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Whittington CF, et al. Individual and Cumulative Effects of GWAS Susceptibility Loci in Lung Cancer: Associations after Sub-Phenotyping for COPD. PLoS One 2011;6:e16476. [Crossref] [PubMed]
- Wilk JB, Shrine NRG, Loehr LR, et al. Genome-Wide Association Studies IdentifyCHRNA5/3andHTR4in the Development of Airflow Obstruction. Am J Respir Crit Care Med 2012;186:622-32. [Crossref] [PubMed]
- Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122. [Crossref] [PubMed]
- Oga T, Nishimura K, Tsukino M, et al. Analysis of the Factors Related to Mortality in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2003;167:544-9. [Crossref] [PubMed]
- Haruna A, Muro S, Nakano Y, et al. CT scan findings of emphysema predict mortality in COPD. Chest 2010;138:635-40. [Crossref] [PubMed]
- Nishimura K, Izumi T, Tsukino M, et al. Dyspnea Is a Better Predictor of 5-Year Survival Than Airway Obstruction in Patients With COPD. Chest 2002;121:1434-40. [Crossref] [PubMed]
- Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J 2013;41:1292-301. [Crossref] [PubMed]
- Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev 2013;22:454-75. [Crossref] [PubMed]
- Agusti A, Sobradillo P, Celli B. Addressing the complexity of chronic obstructive pulmonary disease: from phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am J Respir Crit Care Med 2011;183:1129-37. [Crossref] [PubMed]
- Saji H, Miyazawa T, Sakai H, et al. Survival significance of coexisting chronic obstructive pulmonary disease in patients with early lung cancer after curative surgery. Thorac Cancer 2018;9:19-24. [Crossref] [PubMed]
- Yoshida Y, Kage H, Murakawa T, et al. Worse Prognosis for Stage IA Lung Cancer Patients with Smoking History and More Severe Chronic Obstructive Pulmonary Disease. Ann Thorac Cardiovasc Surg 2015;21:194-200. [Crossref] [PubMed]
- Carr LL, Jacobson S, Lynch DA, et al. Features of COPD as Predictors of Lung Cancer. Chest 2018;153:1326-35. [Crossref] [PubMed]
- Sawa K, Koh Y, Kawaguchi T, et al. PIK3CA mutation as a distinctive genetic feature of non-small cell lung cancer with chronic obstructive pulmonary disease: A comprehensive mutational analysis from a multi-institutional cohort. Lung Cancer 2017;112:96-101. [Crossref] [PubMed]
- Yoneyama R, Aoshiba K, Furukawa K, et al. Nicotine enhances hepatocyte growth factor-mediated lung cancer cell migration by activating the α7 nicotine acetylcholine receptor and phosphoinositide kinase-3-dependent pathway. Oncol Lett 2016;11:673-7. [Crossref] [PubMed]
- Scheffler M, Bos M, Gardizi M, et al. PIK3CA mutations in non-small cell lung cancer (NSCLC): genetic heterogeneity, prognostic impact and incidence of prior malignancies. Oncotarget 2015;6:1315-26. [Crossref] [PubMed]
- Heist RS, Engelman JA. SnapShot: Non-Small Cell Lung Cancer. Cancer Cell 2012;21:448.e2. [Crossref] [PubMed]
- Li S, Li L, Zhu Y, et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer 2014;110:2812-20. [Crossref] [PubMed]
- Chaft JE, Arcila ME, Paik PK, et al. Coexistence of PIK3CA and other oncogene mutations in lung adenocarcinoma-rationale for comprehensive mutation profiling. Mol Cancer Ther 2012;11:485-91. [Crossref] [PubMed]
- Madsen RR, Knox RG, Pearce W, et al. OncogenicPIK3CApromotes cellular stemness in an allele dose-dependent manner. Proc Natl Acad Sci U S A 2019;116:8380-9. [Crossref] [PubMed]
- Cosio BG, Soriano JB, López-Campos JL, et al. Defining the Asthma-COPD Overlap Syndrome in a COPD Cohort. Chest 2016;149:45-52. [Crossref] [PubMed]
- Harada T, Yamasaki A, Fukushima T, et al. Causes of death in patients with asthma and asthma-chronic obstructive pulmonary disease overlap syndrome. Int J Chron Obstruct Pulmon Dis 2015;10:595-602. [PubMed]
- Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J 2005;26:586-93. [Crossref] [PubMed]
- Lin H, Jiang S. Combined pulmonary fibrosis and emphysema (CPFE): an entity different from emphysema or pulmonary fibrosis alone. J Thorac Dis 2015;7:767-79. [PubMed]
- de Torres JP, Marín JM, Casanova C, et al. Lung cancer in patients with chronic obstructive pulmonary disease-- incidence and predicting factors. Am J Respir Crit Care Med 2011;184:913-9. [Crossref] [PubMed]


