
Programmed death-ligand 1 expression discrepancy between primary tumor and metastatic lymph nodes in non-small cell lung cancer
Immune-checkpoint inhibitors (ICI) have recently revolutionized cancer treatment of non-small cell lung cancer (NSCLC). Currently, programmed death-ligand 1 (PD-L1) represents the standard response predictor biomarker for PD-1 or PD-L1 inhibitors. PD-L1 immunohistochemistry staining scoring can be affected by different factors such as the type of antibody, the substrate (tumor cells vs. immune cells), type of tissue (FFPE archive vs. fresh biopsy) and others. Saito et al. reported the PD-L1 staining heterogeneity between primary vs. metastases sites in NSCLC (1).
PD-1 inhibitor, pembrolizumab, as monotherapy is an accepted regiment for patients with PD-L1 >50% as demonstrated in KEYNOTE-24 (2). Furthermore, the current standard-of-care in first-line therapy for advanced NSCLC regardless of PD-L1 status is pembrolizumab in combination with platinum doublet chemotherapy based on the subsequent confirmatory trials; KEYNOTE-189 (non-squamous) and KEYNOTE-407 (squamous) (3,4). Importantly, the PD-L1 ≥50% subgroup has the best response rate to this first-line combination therapy (5).
In order to determine PD-L1 tumor proportion score (TPS) status, 22C3 antibody clone is typically used as a companion diagnostic antibody for pembrolizumab. Meanwhile, 28-8 clone was approved as the complementary diagnostic antibody for nivolumab and atezolizumab. This is also the case for the SP263 assay (6,7). It has become apparent that these tests are now interchangeable; therefore, clinicians are welcome to use any of these tests in the future.
Nonetheless, a complication in establishing accurate TPS status arises when addressing inter- and intra- patient and tumor heterogeneity. So far, the issue of heterogeneity has seldom been discussed in PD-L1 expression in lung cancer. Saito et al. recently produced a report on the discrepancies in tumor PD-L1 status heterogeneity between the primary site and secondary lymph nodes by using two diagnostic assays (22C3, 28-8) (1).
A total of 35 patients with primary tumors and paired lymph node involvement were enrolled in their study, divided into no expression (TPS: <1%), low expression (TPS: 1–49%), or high expression (TPS: ≥50%). Low concordance rate was reported for both 22C3 (28.6%) and 28-8 (31.4%) assays comparing primary tumors with their respective lymph node counterparts. Summarizing, in approximately 70% of the cases, no correlation was found between the TPS status in the primary tumor and metastatic lymph node. Moreover, the study confirms that there is no significant difference between the two assay models (Pearson’s chi-square test: P<0.001).
These findings raise the question of the role of TPS status in guiding ICI treatment decision making in correlation to the biopsy site. The discrepancy is more prominent in the high expressing primary tumors (>50%) for which in comparison with their metastatic lymph nodes presented no or low PD-L1 expression. Indicating that careful assessing should be performed when biopsying only metastatic lymph nodes which may not represent the PD-L1 expression status of the primary tumor. Revealing the importance of a deeper understanding of the lung tumor heterogeneity in order to provide the best treatment strategy.
Previous studies have also emphasized the importance of tumor heterogeneity and the discrepancy between primary and metastatic lesions in different driver mutations and type of cancer, such as in metastatic melanoma carrying BRAF mutation, Valachis et al. reported a discrepancy rate of 13.4% between primary and metastatic lesions in BRAF, while a 7.3% discrepancy was found between two metastatic lesions (8). In breast cancer, the reported receptor status discordance between primary and metastatic lesions in estrogen receptor, progesterone receptor, and HER2 were found to be 17.8, 45.4, and 13.3% respectively (9). In colon cancer the median biomarker concordance rate between primary and metastatic colorectal cancer was 81% (10). In renal cell carcinoma, both PD-1 and PD-L1 expression were higher in primary versus metastatic tumors (11). None of these examples of heterogeneity fall in the discrepancy range found in work conducted by Saito et al. summarized in Table 1.
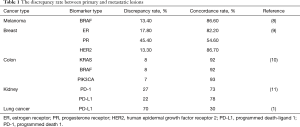
Full table
The current study, as well as previous ones, indicate that biopsy site should be noted in future clinical trials.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.45). NP reports grants and personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Foundation Medicine, Gaurdant360, Genesort, Merk, Merck Sharp & Dohme, Novartis, NovellusDx, Pfizer, Roche, Takeda, other from LOXO. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saito Y, Horiuchi S, Morooka H, et al. Inter-tumor heterogeneity of PD-L1 expression in non-small cell lung cancer. J Thorac Dis 2019;11:4982-91. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non–small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Diker O. Pembrolizumab plus Chemotherapy in Lung Cancer. N Engl J Med 2018;379:e18. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Kian W, Roisman LC, Levitas D, et al. Non-small cell lung cancer PDL1 >50%—should we go single or combo? Precis Cancer Med 2020;3:7. [Crossref]
- Büttner R, Gosney JR, Skov BG, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3867-76. [Crossref] [PubMed]
- Yu H, Boyle TA, Zhou C, et al. PD-L1 Expression in Lung Cancer. J Thorac Oncol 2016;11:964-75. [Crossref] [PubMed]
- Valachis A, Ullenhag GJ. Discrepancy in BRAF status among patients with metastatic malignant melanoma: A meta-analysis. Eur J Cancer 2017;81:106-15. [Crossref] [PubMed]
- Karagöz Özen DS, Ozturk MA, et al. Receptor expression discrepancy between primary and metastatic breast cancer lesions. Oncol Res Treat 2014;37:622-6. [Crossref] [PubMed]
- Bhullar DS, Barriuso J, Mullamitha S, et al. Biomarker concordance between primary colorectal cancer and its metastases. EBioMedicine. 2019;40:363-74. [Crossref] [PubMed]
- Eckel-Passow JE, Ho TH, Serie DJ, et al. Concordance of PD-1 and PD-L1 (B7-H1) in paired primary and metastatic clear cell renal cell carcinoma. Cancer Med 2020;9:1152-60. [Crossref] [PubMed]


