
Younger patients operated for lung cancer have a better prognosis
Introduction
Lung cancer is a leading cause of mortality owing to malignancies in the population of smokers older than 60 years. The median age for primary lung cancer is 70 years old (1) and most papers refer to early lung cancer onset (ELCO) as lung cancer diagnosed before the age of 50 (2). There are conflicting data concerning survival and prognosis in the younger and older lung cancer populations (2-4). Undoubtedly patients with ELCO are a distinct population in comparison with late lung cancer onset (LLCO) patients. There is lower smoking exposure in the ELCO group and the influence of genetic factors has more impact than in LLCO. Carcinoids more commonly occur in the ELCO population compared to more common squamous cell histology in LLCO. Molecular patterns of mutations characterize these groups of patients differently and there are data suggesting a more aggressive course of lung cancer treated at earlier age (3). On the other hand, the younger population is not affected by the number of significant, tobacco-related comorbidities that may influence the overall survival (OS). There are few papers in this field that focus on surgical treatment of early-stage lung cancer solely. The purpose of this study was to compare the differences in survival and prognosis in young and old patients with surgically treated lung cancer.
Methods
Study methods
This is a retrospectively analyzed study made of prospectively collected data of 1,518 patients with lung cancer treated in a Thoracic Surgery Department in the years 2007–2015.
All patients in the study underwent routine preoperative pulmonary evaluation consisting of baseline spirometry. DLCO, predicted postoperative (ppo) spirometry, and ppoDLCO were assessed in selected patients with FEV1% lower than 80%. At that time positron emission tomography (PET) CT was performed in patients with clinical stages cII or higher. Brain CT was undertaken in symptomatic patients and mediastinal staging was performed if indicated by enlarged mediastinal lymph nodes in the mediastinum or if suggested by PET CT.
All patients with coronary heart disease, heart failure or other significant cardiovascular comorbidities were consulted by a cardiologist. Oncological and surgical therapeutic decisions were made during multidisciplinary tumor board meetings. A multidisciplinary team consisting of thoracic surgeons, oncologists, pathologists and radiologists decided treatment options for each patient. A further adjuvant treatment in patients with pathological stages IIA or higher occurred postoperatively, but this data was not collected.
Stage I patients not scheduled for surgical treatment owing to significant comorbidities were accepted to stereotactic radiotherapy program. After the surgery, the patients were surveilled according to routine protocol (follow up every third months during first two years and every six months thereafter). The data were analyzed retrospectively and thus disease-free survival and cancer-specific survival was not registered. The patients in stages higher than IIB were consulted by medical oncologist and considered for adjuvant systemic therapy in cisplatin-based regimen. These data were not recorded and did not enter the analysis.
The operative specimens were not routinely tested for most oncogene mutations like ALK, ROS1, EGFR or others.
The last follow-up visit was recorded in January 2017. The median follow-up was 54 months. Two hundred seventy-five patients died during the study period. No patient data was lost due to incomplete data sampling (i.e., death, withdrawn data consent). The reason of death was not recognized as this data is not accessible from the used national sources.
The death of the patient was obtained using the national registry lead by the Ministry of Internal Affairs and Administration.
Statistical analysis
Including the tumors with histology typical for an age like carcinoids may blur ELCO and LLCO populations comparison. These tumors are characterized by very good long-term prognosis that may a bias in the ELCO group. In order to avoid this, we have performed three different analyses which are listed below.
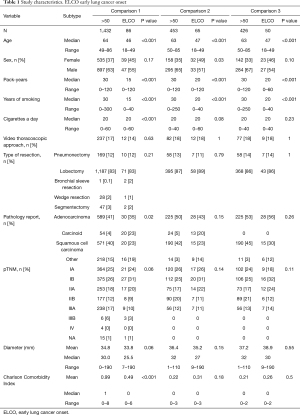
Comparison 1—direct comparison of 86 patients with ELCO were compared to 1,432 patients with LLCO.
Comparison 2—65 patients with ELCO were matched with 453 patients with LLCO in a propensity-score matched analysis (based on exact matching—by sex, pTNM, type of operation, pathological diagnosis and Charlson Comorbidity Index). This study included patients with carcinoid tumors, salivary gland tumors, mucoepidermoid carcinoma, and patients post neoadjuvant treatment and stages IIIB and IV. In each of three comparisons the proportion of males was higher. In this comparison this difference reached the level of statistical significance. We do not find that this incidentally proved trend is discriminating for making conclusions.
Comparison 3—426 patients with LLCO were matched with 50 patients with ELCO in a propensity-score matched analysis (based on exact matching—by sex, pTNM, type of operation, pathological diagnosis and Charlson Comorbidity Index). In this study patients with carcinoid tumors, salivary gland tumors, mucoepidermoid carcinoma, and patients post neoadjuvant treatment and stages IIIB and IV were excluded.
Unpaired data characterized by normal distribution were compared with the unpaired t-test. In the case of non-normal distribution, the Mann-Whitney U-test was applied for comparing two unmatched samples. Categorical variables were assessed by the chi-square test. The accepted level of significance was P<0.05, and hazard ratios (HR) were calculated with 95% confidence interval (CI). In univariable analysis, data were split into two groups: alive or deceased after the end of the study. Those two groups were then compared.
Owing to the nature of this study: a retrospective analysis of prospectively gathered data and the lack of experimental intervention in the study group, university institutional review board accepted the study and decided to waive informed consent (NKBBN/88/2016). Factors which achieved a P value of <0.05 in the univariable analysis entered further assessment via Cox multivariable models of proportional hazards, a multivariable analysis model that makes use of logistic stepwise regression. All variables had P value >0.05 in Schoenfeld Residuals Test meaning the slope of scaled residuals on time is not statistically different from zero, thus, not violating the proportionality assumption.
Results
Comparison 1—in the unmatched population, no differences in gender, pTNM, and type of surgery performed were found. Younger patients were more likely to have typical carcinoid (23.1% vs. 2.6%, P<0.05, OR 11.06, 95% CI, 5.695–21.360) and mucoepidermoid tumours (2.6% vs. 0.3%, P<0.05, OR 7.547, 95% CI, 0.997–44.708), whereas the older patients were more likely to have squamous cell lung carcinoma (39.7% vs. 23.1%, P<0.05, OR 0.455, 95% CI, 0.256–0.799). Younger patients were less likely to be current smokers (18% vs. 41%, P<0.05, OR 0.316, 95% CI, 0.192–0.519). Median Charlson Comorbidity Index in the younger population was 0 and in the older population was 1 (P<0.05). The characteristics of operated patients are presented in Table 1. Five-year survival in patients with ELCO was 71.9% comparing to 58.7% in LLCO patients (log-rank P=0.008) (Figure 1).
Full table
Comparison 2—the propensity score-matched analysis (PSMA) with the exact method including carcinoid tumors, salivary gland tumors, mucoepidermoid carcinoma, and patients post neoadjuvant treatment and stages IIIB and IV; comparing sex, pTNM, type of operation, pathological diagnosis and Charlson Comorbidity Index, showed that younger patients had better survival rates compared to older patients. Five-year survival in patients with ELCO was 77.6% comparing to 61.5% in LLCO patients (P<0.001, HR =0.559, 95% CI, 0.360–0.865) (Figure 2).
Comparison 3—the PSMA with exact method (comparing sex, pTNM, type of operation, pathological diagnosis and Charlson Comorbidity Index), excluding carcinoid tumors, salivary gland tumors, mucoepidermoid carcinoma and patients post neoadjuvant treatment and stages IIIB and IV, showed no significant difference in survival rates comparing ELCO patients with LLCO patients, although there was still a trend towards better survival in ELCO patients (P=0.085, HR =0.607, 95% CI, 0.343–1.073) (Figure 3).
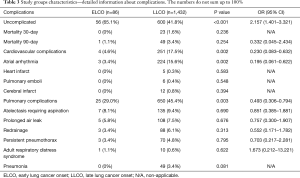
The detailed list of comorbidities is listed in Table 2. Early mortality as well as complications following the surgery are presented in Table 3.

Full table
Full table
Discussion
ELCO population is characterized by different histopathological lung cancer characteristics comparing with LLCO. A high occurrence of neoplasms with relatively low aggressiveness impedes direct analysis of this cohort of patients. The main finding of this study is supporting the thesis that patients with ELCO have better prognosis comparing to LOLC. This trend was observed in the general population of patients operated due to lung cancer. To reduce potential biases we decided to perform two PSMA analyzes.
There are conflicting data concerning the prognosis of patients with ELCO compared to patients with LLCO. While some studies have shown that there is a significantly worse prognosis in the younger population (3) other studies have shown that the prognosis is significantly worse in older patients (2). On the basis of the results of our study, patients with ELCO have higher five-year survival after surgical treatment compared to patients with LLCO.
Bryant and colleagues state in their article that younger patients with surgically resected lung cancer have a significantly worse prognosis compared to older patients (3), which differs greatly from our results. They matched 254 younger patients with 508 older patients. The selection of patients was based on symptoms reported at referral. This study protocol raises selection biases what impairs the ability to conclude rather than relatively small study population. Designing study protocol on vague and often misreported symptoms in the population of patients treated operatively due to early stages of lung cancer may result in a skewed interpretation of obtained data. Our study does not confirm results reported in the mentioned publication. On the other hand, Dell’Amore and colleague presented another paper in 2015 that also compared outcomes in NSCLC in younger and older patients (2). They compared 113 young patients with 347 older patients. They concluded that the OS in young patients is better than in older patients, similar to our data. It was also confirmed by findings of Radzikowska et al. (5) and Tian et al. (6). The above-mentioned reports represent the situation in early stages of lung cancer. The data concerning advanced stages is also conflicting. The results of the analysis of big datasets prove the beneficial effect of younger age in a population of patients with dominating adenocarcinoma in stage III and IV (7,8). Other reports of smaller datasets inform of advanced stage suggest a more aggressive course in younger adults (6,8). In summary, according to most convincing studies young age is a beneficial prognostic factor in both early and advanced stages of lung cancer.
It is difficult to assess what age limit should define the ELCO as there is no definition. Most of the papers define ELCO between 40 and 50 years of age (2-6,9). The advantage of arbitral choice of 50 years of age is supported by relatively high proportion of non-carcinoid tumors in this study group what increases the strengths of the analysis.
Age is not a uniform prognostic factor in different malignancies. What is more, age may be an opposite prognosticator in early and advanced disease. In most subtypes of most common neoplasm in women—breast cancer young age is widely recognized as adverse disease prognosticator (10). Other cancers have similar biology to lung cancer and published studies confirm the results obtained by us. Colorectal cancer is a disease mostly affecting the older population. Wang and colleagues (11) mention that young patients with colorectal cancer had higher stage presentation and more aggressive pathological features, but better survival. This trend in survival was also discovered in the present study. Similarly, in another highly discussed cancer type such as ovarian cancer, Chan and colleagues (12) found that younger patients have better survival compared to their older cohort. Even after adjusting for clinicopathologic prognostic factors the younger patients still had a more favorable outcome and prognosis.
The effect of age on survival is not a result of perioperative risk. Not surprisingly younger patients had less comorbidities (Table 2). It reflects the situation of a pleiotropic effect of tobacco. Many years of cigarette smoking leads not only to increased risk of lung cancer but also chronic obturatory pulmonary disease and arterial hypertension. The population of LLCO has definitely more comorbidities and the postoperative period is characterized by higher level of complications (Table 3). Despite the higher number of both pulmonary and cardiovascular complications they did not affect the 30- and 90-mortality in our study group. It is possible that the trend would be confirmed in an analysis of bigger population. However, on the basis of the analysis we postulate that in general, surgical treatment in EOLC is as safe as in the LOLC. The effect on the survival should not be interpreted as obviously riskier surgical procedure by itself. It should rather be advocated to the biological character of the tumor.
The main obstacle in the interpretation of data obtained directly from the study is the blurring effect of favorable pathology of carcinoids. In order to avoid that we performed two different PSMAs. In fact, we matched our patients regarding sex, pTNM, type of operation, pathological diagnosis and Charlson Comorbidity Index. We decreased potential biases, although not eliminated them completely. In order to completely oust the effect of relatively common tumors of less aggressive behavior, we performed another PSMA after exclusion of carcinoid tumors, advanced cases, rare histologies and patients after neoadjuvant treatment. In this case again we obtained separated survival curves, however, the difference was not significantly significant. The proximity to statistical difference supports the main findings from the study. We presume that observed tendency would convert in a direct effect in a larger population. We highlight the necessity to fully expose the effect of tumors of completely different biology than NSCLC when comparing prognosis in ELCO and LLCO. This is not routinely undertaken and raises a potential bias in several papers (2-4).
The oncogene mutations were not tested routinely in our patients. Adjuvant treatment tailored by the molecular profile of early stage NSCLC was not justified in 2007 when the cohort study started. Even currently it is a matter of controversies. In many cases molecular profile of NSCLC characterizes the clinical course of the disease, the tendency for early intracranial (ALK) or miliary (ROS1) spread. It may be a challenge for clinicians to reschedule the observational algorithm of young patients with driver mutations in resected NSCLC in order to implement systemic treatment as early as needed in case of progression. Additionally it is interesting that the proportion of previous malignancies in ELCO is similar to LLCO despite shorter lifespan before the occurrence of lung cancer. This may suggest the tendency of this population to higher incidence.
There are several limitations to our study. The small number of patients with ELCO without carcinoid may result in improper study samples in order to obtain clear results. This preliminary study showed the necessity of inclusion of a higher patients volume, with an emphasis on collecting a higher amount of data concerning younger patients, from national or international databases. Despite limited number of ELCO patients involved in current study the observed trends were confirmed in statistical analysis what enables making clear clinical conclusions. Another selection bias concerns the limited population of surgical candidates what does not highlight the epidemiological situation of 80% of patients with NSCLC. Another weak point of the paper is the lack of recognition of the reason of death. Analysis of cancer-related deaths brings highest weigh of scientific proof in most of the cases. Nevertheless, we deem to present the present study basing on OS being the objective measure of outcome of oncological treatment. The cut-off value of 50 years of age was not confirmed by a mathematical definition that poses another weak point of the study. This cut-off line, however, enables a comparison of a distinct population of roughly 5% of patients with lung cancer with a rare characteristic.
The clear clinical message of this study is that the clinical aggressiveness of early stage NSCLC in adults younger than 50 years seems to be lower than in older individuals. This should encourage to wider involvement of this patients in the radical treatment. Local treatment should be considered in higher risk patients like stage III patients or selected population of individuals with oligometastatic disease. This statement is not clearly supported by the evidence gathered, but it may guide the directions for the future studies.
Conclusions
Our study has shown that younger patients may have better OS compared to older patients. In the future, studies should take into consideration that including neuroendocrine tumors such as typical and atypical carcinoid tumors might skew the result as these tumors behave differently compared to the more prominent lung cancers.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.55). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. A retrospective analysis of prospectively gathered data and the lack of experimental intervention in the study group, university institutional review board accepted the study and decided to waive informed consent (NKBBN/88/2016).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Cancer Institute. (n.d.). Cancer Stat Facts: Lung and Bronchus Cancer. Available online: (accessed February 2, 2019).https://seer.cancer.gov/statfacts/html/lungb.html
- Dell'Amore A, Monteverde M, Martucci N, et al. Surgery for non-small cell lung cancer in younger patients: what are the differences? Heart Lung Circ 2015;24:62-8. [Crossref] [PubMed]
- Bryant AS, Cerfolio RJ. Differences in outcomes between younger and older patients with non-small cell lung cancer. Ann Thorac Surg 2008;85:1735-9; discussion 1739.
- Skarin AT, Herbst RS, Leong TL, et al. Lung cancer in patients under age 40. Lung Cancer 2001;32:255-64. [Crossref] [PubMed]
- Radzikowska E, Roszkowski K, Głaz P. Lung cancer in patients under 50 years old. Lung Cancer 2001;33:203-11. [Crossref] [PubMed]
- Tian DL, Liu HX, Zhang L, et al. Surgery for young patients with lung cancer. Lung Cancer 2003;42:215-20. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Etzel CJ, Lu M, Merriman K, et al. An epidemiologic study of early onset lung cancer. Lung Cancer 2006;52:129-34. [Crossref] [PubMed]
- Lara MS, Brunson A, Wun T, et al. Predictors of survival for younger patients less than 50 years of age with non-small cell lung cancer (NSCLC): a California Cancer Registry analysis. Lung Cancer 2014;85:264-9. [Crossref] [PubMed]
- Liedtke C, Rody A, Gluz O, et al. The prognostic impact of age in different molecular subtypes of breast cancer. Breast Cancer Res Treat 2015;152:667-73. [Crossref] [PubMed]
- Wang R, Wang MJ, Ping J. Clinicopathological Features and Survival Outcomes of Colorectal Cancer in Young Versus Elderly: A Population-Based Cohort Study of SEER 9 Registries Data (1988-2011). Medicine (Baltimore) 2015;94:e1402. [Crossref] [PubMed]
- Chan JK, Urban R, Cheung MK, et al. Ovarian cancer in younger vs older women: a population-based analysis. Br J Cancer 2006;95:1314-20. [Crossref] [PubMed]





