
Comparison of radiopaque dye materials for localization of pulmonary nodules before video-assisted thoracic surgery
Introduction
Video-assisted thoracic surgery (VATS) is increasingly being performed for partial lobectomy for pulmonary nodules because it is less invasive than thoracotomy and offers the possibility of therapeutic diagnosis (1,2). However, because small or ground-grass opacity nodules are difficult to detect on thoracoscopy, localization is required prior to VATS (3,4). Several percutaneous localization techniques have been reported, including hook-wire insertion (5-7), radionuclide injection (7,8), contrast medium (lipiodol or barium) injection (9-11), and dye injection (12-17). However, these each have advantages and disadvantages, and there is no consensus regarding which technique is superior for lung nodule localization.
Recent studies have reported the effectiveness of lung nodule localization using a mixture of dye material and contrast medium (18,19). The injection point of the material can be detected on thoracoscopy by dye pigmentation on the lung surface. In the event that the pigmentation is unclear, the injection point can be confirmed using intraoperative fluoroscopy because the mixture is radiopaque. However, the optimal combination of dye and contrast medium for this technique is unknown. In addition, a previous study has reported the usefulness of adding lidocaine gel to the mixture (19,20), but the advantages of doing so have not been well investigated.
The aim of this study was to evaluate and compare the characteristics of various combinations of pigments and radiopaque materials and determine the most suitable type of material for localization of pulmonary nodules prior to VATS.
Methods
Samples
The following materials were used in the study: Indigo carmine (Indigocarmine; Daiichi Sankyo Co. Ltd., Tokyo, Japan), indocyanine green (ICG) (Diagnogreen; Daiichi Sankyo Co. Ltd.), lipiodol (Lipiodol; Guerbet Japan K.K., Tokyo, Japan), nonionic iodine contrast medium (Iopamiron 300; Bayer Yakuhin, Ltd., Osaka, Japan) and lidocaine gel (Xylocaine Jelly 2%; Aspen Japan K.K., Tokyo, Japan). ICG was prepared as 1% by dissolving 25 mg of ICG with 2.5 mL distilled water (w-ICG) or with contrast medium (cm-ICG).
We compared six radiopaque dye materials: (I) 1:1 mixture of indigo carmine and lipiodol; (II) 2:2:1 mixture of indigo carmine, lipiodol, and lidocaine gel; (III) 1:1 mixture of w-ICG and lipiodol; (IV) 2:2:1 mixture of w-ICG, lipiodol, and lidocaine gel; (V) cm-ICG; and (VI) 4:1 mixture of cm-ICG and lidocaine gel. Each mixture was created using a three-way stopcock and two syringes.
Measurement of liquid characteristics
Stability was evaluated by observing changes in the mixtures in the test tube with time. For each liquid material, 5 mL were added to a test tube, which was photographed immediately and at 15 min, 30 min, 45 min, 1 h, 3 h, 12 h, 1 day, and 2 days after preparation. An X-ray image was taken using a fluoroscopic apparatus (Infinix Celeve-I INFX-8000C; Canon Medical Systems Corp., Otawara, Japan) at the same timings as the photographs.
Viscosity was evaluated using a rotational viscometer (Viscotester VT-06; RION Co. Ltd., Kokubunji, Japan). During the experiments, the room temperature was maintained at 25 °C.
Visibility in the lung phantom
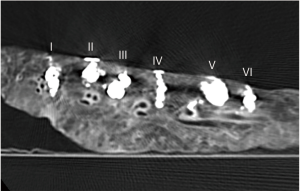
We compared the visibility of each liquid material after its injection into a lung phantom. For each material, a 23-gauge needle (Terumo Cattelan Needle; Terumo Corp., Tokyo, Japan) was inserted 1 cm into a pig lung phantom that had been expanded via an intubation tube under CT fluoroscopic guidance (Aquilion LB; Canon Medical Systems Corp., Otawara, Japan) and 0.5 mL of the material was injected while withdrawing the needle until the material reached to the lung surface. The CT image was obtained immediately after injection. The area of the injected material was measured on the slice showing the widest extent.
The detectability of the injection point on the lung surface was evaluated with normal thoracoscopic imaging and near-infrared fluorescence imaging (Image1 STM; Karl Storz SE & Co. KG, Tuttlingen, Germany).
Statistical analysis
The viscosity of each material was measured five times, and each material was injected five times into the pig lung phantom. Data are expressed as the mean ± standard deviation. The correlation between viscosity and area in the pig lung phantom was calculated by correlation coefficients. The correlation was defined as very weak, weak, moderate, strong, and very strong when the correlation coefficient value was 0≤r<0.2, 0.2≤r<0.4, 0.4≤r<0.7, 0.7≤r<0.9, and 0.9≤r<1.0, respectively. Statistical analyses were performed using SPSS software (SPSS for Windows, version 24; IBM, Armonk, NY, USA).
Results
Characteristics of the liquids
Photographs and X-ray images of the liquid materials in test tubes are shown in Figures 1 and 2, respectively. Separation could be seen 15 min after preparation in (I) and (III), and 1 h after preparation in (II), both visually and radiographically. In (IV), separation could be seen on the photographs but not on the X-ray images from 3 h after preparation. (V) and (VI) showed no changes within the 2-day observation period.
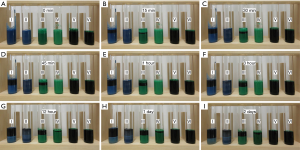

The viscosities of the liquid materials were 0.2±0.1 for (I), 2.9±0.1 for (II), 0.2±0.1 for (III), 2.6±0.1 for (IV), 0.2±0.1 for (V), and 1.2±0.1 dPa·s for (VI).
Visibility in the lung phantom
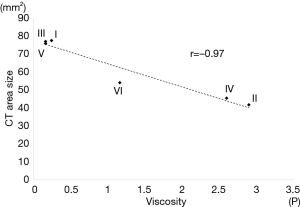
All materials were visible as areas of high attenuation on the CT images of the lung phantom obtained after injection of the liquid materials (Figure 3). The mean areas of the materials in the CT slices that showed the widest areas were 77.5±21.7 for (I), 41.8±3.4 for (II), 77.0±15.5 for (III), 45.5±3.5 for (IV), 75.9±14.0 for (V), and 54.1±10.5 mm2 for (VI). There was very strong negative correlation between viscosity and area in the pig phantom (r=−0.97, Figure 4)
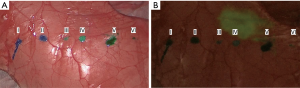
The puncture point of each material could be detected easily on the thoracoscopic image (Figure 5A). On near-infrared fluorescence imaging, ICG fluorescence was not detected at any injection point, even for materials containing ICG (Figure 5B).
Discussion
The differences found among the radiopaque dye materials evaluated in the present study may assist in selecting the optimal material for localizing pulmonary nodules prior to VATS.
The mixture of indigo carmine and lipiodol and the mixture of w-ICG and lipiodol separated in the test tube only 15 minutes after preparation. Although it is best to perform preoperative localization immediately before surgery because the dye becomes less detectable with time (19), the timing of the localization procedure can be delayed by the availability of the CT and surgery rooms. Sometimes, the procedure must be performed several days before surgery. Thus, stability is an important quality for the material used to localize pulmonary nodules. Recently Seol et al. reported that the proportion of indigo carmine, lipiodol, and lidocaine gel of 5:5:2 was most stabilized (20). We also experimented with not exactly the same but similar proportion of 2:2:1 and showed good stability. Thus, these proportions seem to be ideal to create mixture for preoperative marking.
The pig phantom experiment showed very strong negative correlation between viscosity and area. In other words, by using highly viscous materials, diffusion is suppressed and localization is more likely to be successful. In addition, high viscosity may act to prevent the flow of liquid materials into the vessels. Yoshida et al. reported an instance of flow of the mixture of dye and contrast medium into the vessels at the time of percutaneous lung nodule localization (21). To the best of our knowledge, no studies have reported ischemic complications following lung nodule localization using liquid materials, but lipiodol migration to the brain has been reported after trans-arterial chemoembolization or lymphatic embolization using lipiodol (22,23). Use of high viscosity materials might prevent migration and ischemic complications.
In this study, the puncture point of each material in the pig lung phantom could be detected easily. However, as there was no blood flow or respiratory motion in the phantom, the puncture and injection might have been easier than in a clinical localization procedure. Moreover, as we evaluated the appearance immediately after injection, diffusion might not have advanced in this short time frame.
Indigo carmine and methylene blue are difficult to detect on lungs with severe anthracosis, in which case they show as black or dark color tones (12,14). ICG is reported to be effective for lung surface marking in this condition because of its green color (14-17). The color tones of the mixtures of w-ICG and lipiodol (III) and of w-ICG, lipiodol, and lidocaine gel (IV) were brighter than the other materials on thoracoscopy after injection in the pig lung phantom. We hypothesize that these mixtures might be effective also in lungs with severe anthracosis. Further investigations should include evaluation of the appearance of the surface markers after injection in human lung.
Near-infrared fluorescence imaging could not detect ICG fluorescence at the injection point in the materials containing ICG. Anayama et al. reported the effectiveness of near-infrared fluorescence imaging in percutaneous localization using ICG (15). As ICG shows fluorescence after binding with plasma proteins such as albumin or lipoprotein (24), the fluorescence might not have shown in our lung phantom because there was no blood or lymph flow. The usefulness of near-infrared fluorescence imaging with materials containing ICG should also be evaluated in future.
This study has several limitations. First, because we used a pig lung phantom, we could not evaluate the effects of blood or lymph flow, or respiratory movement. Vital reactions were also unclear. As there are some metabolic differences between humans and pigs, the materials might show an altered appearance in human lung. Second, as we did not observe the lung phantom within the thoracic cavity, we could not evaluate the actual appearance on VATS. Third, the examination was performed at room temperature (25 °C). The characteristics of the liquids might have been different than at body temperature. Fourth, we evaluated only the selected materials and mix proportions. Our selection was based on costs, availability, and technical simplicity; however, it is not impossible that there might be more effective materials and mix proportions. Despite these limitations, the present study revealed various characteristics of radiopaque dye materials, which will be useful for selecting the optimal materials for use in localization of pulmonary nodules prior to VATS.
Acknowledgments
Funding: This work was supported by the Aichi Cancer Research Foundation.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-4057). TH reports grants from Aichi Cancer Research Foundation, during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143e142S-e165S.
- Congregado M, Merchan RJ, Gallardo G, et al. Video-assisted thoracic surgery (VATS) lobectomy: 13 years' experience. Surg Endosc 2008;22:1852-7. [Crossref] [PubMed]
- Nakashima S, Watanabe A, Obama T, et al. Need for preoperative computed tomography-guided localization in video-assisted thoracoscopic surgery pulmonary resections of metastatic pulmonary nodules. Ann Thorac Surg 2010;89:212-8. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: A systematic review and meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Suzuki K, Shimohira M, Hashizume T, et al. Usefulness of CT-guided hookwire marking before video-assisted thoracoscopic surgery for small pulmonary lesions. J Med Imaging Radiat Oncol 2014;58:657-62. [Crossref] [PubMed]
- Iguchi T, Hiraki T, Gobara H, et al. CT fluoroscopy-guided preoperative short hook wire placement for small pulmonary lesions: evaluation of safety and identification of risk factors for pneumothorax. Eur Radiol 2016;26:114-21. [Crossref] [PubMed]
- Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg 2007;32:843-7. [Crossref] [PubMed]
- Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. [Crossref] [PubMed]
- Mogi A, Yajima T, Tomizawa K, et al. Video-assisted thoracoscopic surgery after preoperative CT-guided lipiodol marking of small or impalpable pulmonary nodules. Ann Thorac Cardiovasc Surg 2015;21:435-9. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Lee NK, Park CM, Kang CH, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol 2012;13:694-701. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Shimamura Y, Sasaki S, Shimohira M, et al. New technique of percutaneous CT fluoroscopy-guided marking before video-assisted thoracoscopic surgery for small lung lesions: feasibility of using a 25-gauge needle without local anaesthesia. Br J Radiol 2018;91:20170692. [Crossref] [PubMed]
- Chino S, Kuriyama K, Isohashi K, et al. Percutaneous localization of pulmonary nodules with CT guidance for lung resection: use of dyes. Nihon Igaku Hoshasen Gakkai Zasshi 2003;63:308-10. [PubMed]
- Anayama T, Hirohashi K, Miyazaki R, et al. Near-infrared dye marking for thoracoscopic resection of small-sized pulmonary nodules: comparison of percutaneous and bronchoscopic injection techniques. J Cardiothorac Surg 2018;13:5. [Crossref] [PubMed]
- Nagai K, Kuriyama K, Inoue A, et al. Computed tomography-guided preoperative localization of small lung nodules with indocyanine green. Acta Radiol 2018;59:830-5. [Crossref] [PubMed]
- Zhang C, Lin H, Fu R, et al. Application of indocyanine green fluorescence for precision sublobar resection. Thorac Cancer 2019;10:624-30. [Crossref] [PubMed]
- Kim KS, Beck KS, Lee KY, et al. CT localization for a patient with a ground-glass opacity pulmonary nodule expecting thoracoscopy: a mixture of lipiodol and India ink. J Thorac Dis 2017;9:E349-E353. [Crossref] [PubMed]
- Hasegawa T, Kuroda H, Sato Y, et al. The utility of indigo carmine and lipiodol mixture for preoperative pulmonary nodule localization before video-assisted thoracic surgery. J Vasc Interv Radiol 2019;30:446-52. [Crossref] [PubMed]
- Seol HY, Ahn HY, Chung HS, et al. Appropriate amounts proportions of lidocaine gel, indigo carmine and lipiodol mixture for preoperative marking in video-assisted thoracic surgery. Gen Thorac Cardiovasc Surg 2020. [Crossref] [PubMed]
- Yoshida R, Yoshizako T, Nakamura M, et al. Nonfatal air embolism complicating percutaneous CT-guided lung biopsy and VATS marking: Four cases from a single institution. Clin Imaging 2018;48:127-30. [Crossref] [PubMed]
- Zach V, Rapaport B, Yoo JY, et al. Multiple ischemic strokes after transcatheter arterial chemoembolization for hepatocellular carcinoma with a radiographic and pathological correlate. J Stroke Cerebrovasc Dis 2012;21:217-24. [Crossref] [PubMed]
- Kirschen MP, Dori Y, Itkin M, et al. Cerebral lipiodol embolism after lymphatic embolization for plastic bronchitis. J Pediatr 2016;176:200-3. [Crossref] [PubMed]
- Ohnishi S, Lomnes SJ, Laurence RG, et al. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging 2005;4:172-81. [Crossref] [PubMed]


