
Endovascular aortic repairs combined with looping-chimney technique for repairing aortic arch lesions and reconstructing left common carotid artery
Introduction
Thoracic endovascular aortic repair (TEVAR) has been used as a solid alternative to conventional open surgery for many patients suffering from thoracic aortic diseases. It is most commonly used to treat descending thoracic aortic aneurysms (TAAs) and type B aortic dissection (TBAD). Core principle underpinning is to place a covered stent graft over the lesions in the descending thoracic aorta. But for aortic arch pathologies, it is usually difficult to treated with open surgery (1), and it is a challenge of TEVAR, because of insufficient (<15 mm) proximal landing zone (PLZ) and revascularization of the supra-aortic branches (SAB). Both effective exclusion of lesion and restoration of blood flow to the brain are primary concern for patients with thoracic aortic arch pathologies.
In recent years, with the rapid progress of endovascular treatment, more and more attempts have been made to apply TEVAR to treat aortic arch diseases. The commonly used techniques for SAB rebuilding include Hybrid surgery, chimney graft (CG), grafts fenestration and branch stent. However, due to their own limitations, these techniques are only applied in patients who meet strict anatomic criteria.
CG technique, which is introduced to rescue accidentally covered aortic branches during aortic endovascular repair, has been most commonly used to reconstruct left subclavian artery (LSA); nevertheless, this method is limited in left common carotid artery (LCCA) and has not been regular applying, since it requires surgical exposure or percutaneous carotid artery access, which may further complicate the procedure, prolong the operation time, require blocking of the carotid artery, and increase the risk of central nervous system events. Catheter looping at ascending aorta has been used for carotid angiographic examination and stenting (2-5). Nevertheless, no studies investigated the application of catheter looping for endovascular treatment of aortic disease so far. In this study, a minimally invasive retrograde brachial access—looping chimney technique (LCT) was applied via percutaneous right brachial artery (RBA) access to reconstruct the LCCA. The aim of this retrospective research was to evaluate the feasibility and efficacy of TEVAR combined with LCT for the remedy of the aortic diseases.
Methods
Patients
Between December 2016 and December 2018, patients presenting with aortic arch pathologies were evaluated for therapy including open surgery, hybrid surgery, or TEVAR. Computed tomography angiography (CTA) was used to verify the diagnosis and morphology of the pathology, including the evaluation of the bilateral carotid, subclavian, and vertebral arteries and the circle of Willis. Patients with the following anatomic criteria have been selected as candidates for the LCT in the LCCA: PLZ involving zone 0 or zone 1; patent bilateral carotid and vertebral arteries as well as the circle of Willis; and dominant right vertebral artery. Patients with IA diameter >12 mm, PLZ diameter >40 mm, and dissection of the right common carotid artery or the right subclavian artery were excluded from the study. Finally, a total of 14 patients (mean 52.86±14.46; range, 27–79; 10 men, 4 women) with aortic arch pathologies underwent TEVAR associated with LCT to reconstruct the LCCA.
Treatment procedure
TEVAR was performed in a digital subtraction angiography (DSA) operating room. Local anesthesia was applied before surgery. DSA of the aorta was used to compare the morphologic and dynamic characteristics observed by CT scan. LCT was performed before regular TEVAR operation.
A percutaneous RBA access was established with an 8F arterial sheath (Terumo, Tokyo, Japan) at first. Then, a 0.035-inch, 260-cm, J-tip guidewire (RADIFOCUS, TERUMO, Japan) was positioned into the left external carotid artery after looping in the aortic sinus. A 5-F, 125-cm, VERT impress® catheters (MeritMedical, Utah, USA) was introduced upward into the left external carotid artery and was reserved, which was inserted into a 6-F, 90-cm, carotid guiding sheath (Destination®, Terumo, Maryland, USA) or a 8-F, 90-cm, MPA1 guiding sheath (Vista Brite Tip®, Cordis, Florida, USA) in advance. Next, to exchange the guidewire with a 0.035-inch, 260-cm, J-tip stiff guidewire (RADIFOCUS®, Terumo, Tokyo, Japan), and the guiding sheath was introduced passing the loop through the catheter and guidewire, and was reserved into the LCCA after withdrawing the catheter and guidewire. It is notable that the end of the loop should be kept on the aortic valve to avoid loop or guidewire escaping from the LCCA.
After aortic stent graft was positioned in the aortic arch, chimney stent grafts were implanted into the LCCA and/or IA from percutaneous RBA access, and the chimney stent grafts were placed parallel to the aortic stent graft, between proximal end and covered part of aortic stent graft. Using a balloon to optimize chimney stent graft expansion was performed to mold LCCA and/or IA. At last, DSA was done to confirm the procedure effectiveness. For the patients with superior right vertebral artery, no carotid stenosis, and intact Willis ring, planned coverage of LSA was performed, while for those with superior left vertebral artery, fenestration in situ was used to rebuilt LSA, and these patients were excluded from the study. The primary success of the surgery was defined as: (I) primary aortic pathologies was effectively excluded, (II) no endoleak, (III) SABs were patent.
Follow-up
All patients underwent CTA 2 weeks after surgery and were followed up for 3, 6 and 12 months. Each visit included physical examination and CTA. Telephone follow-up was performed semiannually.
Statistical analysis
Statistical analysis was performed with SPSS version 22.0. Means and standard deviation (SD) or median and range were reported for parametric data; and absolute values and percentages were reported for nonparametric data.
Ethical approval
Prior to surgery, an interdisciplinary committee, including vascular surgeons, cardiovascular surgeons, and anesthesiologists, discussed all cases. And the study was approved by ethics board of Yan’an Affiliated Hospital of Kunming Medical University (No. 2020-004-01).
Results
Procedures
From December 2016 to December 2018, 14 patients (10 male, 4 female; average age 52.86±14.46; range, 27–79 years) with aortic arch pathologies underwent the endovascular operation. Pathologies included type B aortic dissection (n=8), penetrating aortic ulcers (n=1), retrograde type A aortic dissection (n=1), thoracic aortic aneurysm (n=2), thoracic aortic pseudoaneurysm (n=2). The patients’ overall clinical demographics, characteristics and comorbidities are summarized in Table 1.

Full table
LCT was used to successfully reconstruct the LCCA in all 14 patients (100%). In one patient (patient 11) with TBAD, a percutaneous revascularization of IA was performed following CG implantation of LCCA via RBA. The endografts were positioned in zone 1 (Ishimaru classification, n=12) and zone 0 (n=2). LSA of 14 patients were intentionally covered. Sixteen aortic stent grafts [Ankura (Lifetech Scientific Co, Ltd., Shenzhen, China), Hercules (MicroPort Medical Co, Ltd., Shanghai, China)] were deployed in the aorta and Absolute Pro (Abbott Vascular, Abbott Park, IL, USA), Omnilink Elite (Abbott Vascular, Abbott Park, IL, USA), complete SE (Medtronic Vascular, Santa Rosa, CA, USA), and Protégé GPS (ev3, USA) were chimney stent grafts that were used. All Stents and other endografts characteristics are summarized in Table 2.
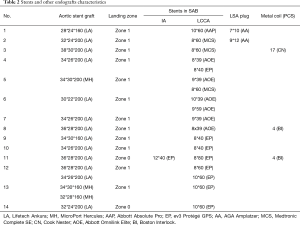
Full table
Perioperative outcomes
Type Ia endoleak occurred in 3 patients during the procedure; one of them was TAA (Figure 1), other two were TBAD. For these three patients, metal coils (Boston Scientific Inc., Natick, MA, USA) were used to decrease the endoleak. In two patients with type II endoleak, the LSA was blocked by Amplatzer II plug (AGA Medical Corporation, Plymouth, MN, USA); one case was thoracic aortic pseudoaneurysm and the other was type B aortic dissection (Figure 2). In 4 patients, the chimney stent of LCCA was partially squeezed and another bare stent was implanted to strengthen the radial support force. One patient who had abnormal coagulation function died 3 days after surgery due to cerebral hemorrhage.
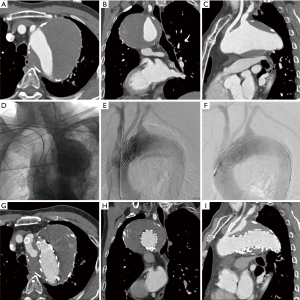

Short-term follow-up
The mean follow-up duration was 9.77±6.64 months (range, 0–24). With the exception of one dead case, all 13 patients survived the follow-up period and had no further complications.
The three patients with type Ia endoleak and two patients with type II endoleak, had no further endoleaks occurring during follow-up, and remained asymptomatic. One patient had a transient blurred vision. There were no obvious abnormalities in the head CT and he recovered spontaneously on the third day after surgery. One IA and thirteen LCCA restored by LCT, and four LSAs rebuilt by in situ fenestration were all patent. There was no stent migration, deformation, fracture, no thrombosis in the stent, and no compression on the chimney stent, indicated by final CT follow-up.
Discussion
In the treatment of aortic disease involving SAB or with short PLZ, the commonly used techniques are traditional thoracotomy, Hybrid surgery, CG, grafts fenestration and branch stent. Nevertheless, all these techniques have some unsatisfactory aspects in clinical application. Traditional thoracotomy is technically difficult and traumatic, and can cause complications (6), with mortality and central nervous system events that occur in 29% and 18% cases, respectively (7,8). Hybrid surgery is not totally a noninvasive method, because of preoperative extra-anatomical bypass or intraoperative transposition, it has been associated with risk of iatrogenic injury and complications; the incidence of perioperative mortality and central nervous system events have been reported to reach 11.9% and 7.6%, respectively (9).
Grafts fenestration and branch stent has been restricted because of unformed equipment, technical requirements, complexity, customization, and increased risk of neurological complications caused by complex manipulations on the arch (10-12). Therefore, it is urgently required to investigate the new endovascular methods which could both extend PLZ and restore SAB for particular patients. In this group, all patients who had insufficient PLZ (11 patients had lesions involving LSA) underwent totally endovascular surgical therapy. And all 14 LSAs were intentionally covered. Because they have superior right vertebral artery and intact Willis ring, that does not significantly increase the risk of central nervous system ischemia (13).
Sometimes, LCCA and IA were inadvertently covered by aortic graft stent, and it would be disastrous that if cephalic perfusion cannot be promptly restored by emergency carotid incision or bypass surgery, and it will complicate the procedure, prolong the operation time and require blocking of the carotid artery (14-28).
Therefore, LCT as a kind of CG technique, it has strong technical feasibility, and causes lower rate of perioperative mortality, endoleak incidence, and central nervous system events (23,29,30). As a new way to reconstruct LCCA through totally endovascular approach. Guidewire and catheters were pushed into LCCA via percutaneous RBA access by looping in the ascending aorta before aorta stent graft deployment. In our series, a total of 18 CGs were implanted in 14 patients’ LCCA through LCT, including 1 patient with double chimneys in both IA and LCCA. Type Ia endoleak occurred instantly after TEVAR in 3 patients, but it was effectively eliminated by metal coil filling. Similarly, two patients with type II endoleak were treated directly and effectively by plugging the LSA. In the other three patients, CG in LCCA was compressed partially, and then efficaciously expanded by re-implanting a bare stent. No combinations (endoleak, compression of stent grafts, or other complications) were observed in 13 patients; one IA and thirteen LCCA restored by LCT, and four LSAs rebuilt by in situ fenestration were all patent; except in one patient who died of cerebral hemorrhage due to abnormal coagulation function. It has obvious advantages compared with other branch revascularization procedures. The highlights of the procedure were: (I) PLZ was effectively extended; (II) vital SABs were preserved using off-the-shelf devices; (III) there was no need for surgical exposure and puncture of carotid artery, which did not affect the blood flow to the brain; (IV) more importantly, in case of inadvertent complete or partial coverage of LCCA and/or IA, CGs can be implanted timely through the reserved looping guidewire.
Whereas, deficiencies existing in conventional chimney technique are also inevitable in LCT. Firstly, there is channel between the aortic endograft, CG and aorta intima, thus CG may increase the risk of type Ia endoleak, especially in patients whose primary entry tear is close to LSA orifice; in patients whose tear is large on the greater curvature of the aortic arch; and in patients whose LSA is perpendicular to the aortic arch (31). The leptosomatic gutter supplies a high-flow impedance circumstance and blood flow in the gutter is significantly slowed down and forms vortices (26), so proper overlapping length can induce thrombosis in the gutter (32,33), 2–5 cm overlapping can reduce gutter-related type I endoleaks (25,34-36). In this study, the overlapping was treated as the proximal end of CG transcending the proximal end of covered stent, which did not exceed the range of the bare stent of the aortic endograft. Secondly, radial support force involving the aortic stent and the CG is different, which may affect not only the long-term patency rate of the CG but also certain complications, such as type Ia endoleak, compression of chimney stent graft, or retrograde type A aortic dissection (37,38). The outcome may depend on type and size of stents. In our group, Ankura (Lifetech, n=13) and Hercules (MicroPort, n=3) were deployed in the aorta; while Absolute Pro (Abbott, n=1), Omnilink Elite (Abbott, n=6), Complete SE (Medtronic, n=3), and Protégé GPS (ev3, n=9) were used for CG. Stents with 10–15% oversize and those with 5–10% oversize diameter were used for PLZ and for SAB, respectively.
It is noteworthy that medicine administration is essential in terms of assuring the success of treatment and prognosis. Anticoagulation or antiplatelet may be beneficial for long-term patency and long-term prognosis. A study (39) recommend postoperative clopidogrel (75 mg/d) for 1 month and aspirin (325 mg/d) for life, considering that thrombosis in the gutter could be the source of emboli to the brain (32,40). In our study, all patients received low-molecular-weight heparin (4,000 IU/Qd) for 5–7 days, and were prescribed oral clopidogrel (75 mg/d) and aspirin (100 mg/d) for 6 months at least.
The study has some limitations. As retrospective feasibility study, we believed that 14 patients were fair enough for this technique, but ten LSAs were sacrificed, we were supposed to rebuild them even if they have not been getting any combinations and complications by now. We did not compare within groups sorted by diseases and with approaches applied currently. And prospective randomized controlled studies and more samples are still required to get more rigorous data. It is noteworthy that this kind of research is difficult to avoid bias because of anatomical variations and lesions complexity of aortic arch.
Conclusions
LCT combined with TEVAR is an endovascular alternative and safe procedure, which can be used to preserve LCCA and/or innominate artery (IA) via percutaneous RBA access in high-risk patients who cannot undergo open surgery or hybrid surgery. Endoleak, compression of chimney stent graft and central nervous system events are the main perioperative concerns. Hence, more long-term studies are necessary to evaluate the durability and effectiveness of this innovative endovascular procedure.
Acknowledgments
Funding: This work was supported by the Technology and Welfare Planning Commission of Yunnan Province Grant (grant number 2014RA071), United Grant of Yunnan Provincial Science and Technology Department and Kunming Medical University (grant number 2015FB085 and 2017FE468-090).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.31). JZ reports grants from Yunnan Provincial Science and Technology Department, during the conduct of the study; XL reports grants from Yunnan Provincial Science and Technology Department, grants from Kunming Medical University, during the conduct of the study; MT reports grants from Yunnan Provincial Science and Technology Department, grants from Kunming Medical University, during the conduct of the study; HC reports grants from Yunnan Provincial Science and Technology Department, during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by ethics board of Yan’an Affiliated Hospital of Kunming Medical University (No. 2020-004-01).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Safi HJ, Rd MC, Estrera AL, et al. Optimization of aortic arch replacement: two-stage approach. Ann Thorac Surg 2007;83:S815-8. [Crossref] [PubMed]
- Fang HY, Chung SY, Sun CK, et al. Transradial and transbrachial arterial approach for simultaneous carotid angiographic examination and stenting using catheter looping and retrograde engagement technique. Ann Vasc Surg 2010;24:670-9. [Crossref] [PubMed]
- Ruzsa Z, Nemes B, Pintér L, et al. A randomised comparison of transradial and transfemoral approach for carotid artery stenting: RADCAR (RADial access for CARotid artery stenting) study. Eurointervention 2014;10:381-91. [Crossref] [PubMed]
- Ruzsa Z, Sasko K. Transradial/Transbrachial Carotid Artery Stenting With Proximal or Distal Protection: A Promising Technique for the Reduction of Vascular Complications and Stroke. J Endovasc Ther 2016;23:561-5. [Crossref] [PubMed]
- Lee WC, Fang HY, Chen HC, et al. Comparison of a Sheathless Transradial Access With Looping Technique and Transbrachial Access for Carotid Artery Stenting. J Endovasc Ther 2016;23:516. [Crossref] [PubMed]
- Sun L, Qi R, Chang Q, et al. Surgery for acute type A dissection with the tear in the descending aorta using a stented elephant trunk procedure. Ann Thorac Surg 2009;87:1177-80. [Crossref] [PubMed]
- Patel HJ, Nguyen C, Diener AC. Open arch reconstruction in the endovascular era: Analysis of 721 patients over 17 years. J Thorac Cardiovasc Surg 2011;141:1417. [Crossref] [PubMed]
- Hiraoka A, Chikazawa G, Tamura K, et al. Clinical outcomes of different approaches to aortic arch disease. J Vasc Surg 2015;61:88-95. [Crossref] [PubMed]
- Moulakakis KG, Mylonas SN, Markatis F, et al. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg 2013;2:247. [PubMed]
- Brar R, Ali T, Morgan R, et al. Endovascular repair of an aortic arch aneurysm using a branched-stent graft. Eur J Vasc Endovasc Surg 2008;36:545-9. [Crossref] [PubMed]
- Wipper S, Lohrenz C, Ahlbrecht O, et al. Antegrade side branch access in branched aortic arch endografts: a porcine feasibility study. J Endovasc Ther 2013;20:233-41. [Crossref] [PubMed]
- Lioupis C, Corriveau MM, Mackenzie KS, et al. Treatment of Aortic Arch Aneurysms with a Modular Transfemoral Multibranched Stent Graft: Initial Experience. Eur J Vasc Endovasc Surg 2012;43:525. [Crossref] [PubMed]
- Rizvi AZ, Murad MH, Fairman RM. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: A systematic review and meta-analysis. J Vasc Surg 2009;50:1159-69. [Crossref] [PubMed]
- Wang L, Huang Y, Guo D, et al. Application of triple-chimney technique using C-TAG and Viabahn or Excluder iliac extension in TEVAR treatment of aortic arch dilation diseases. J Thorac Dis 2018;10:3783-90. [Crossref] [PubMed]
- Fallatah R, Elasfar AA, Alzubaidi S, et al. Endovascular Repair of a Leaking Aortic Arch Pseudoaneurysm Using Graft Stent Combined with Chimney Protection to Left Common Carotid Artery: Case Report and Review of Literature. J Saudi Heart Assoc 2018;30:254-9. [Crossref] [PubMed]
- Zhao Y, Cui J, Yin H, et al. Hybrid operation versus the chimney technique for reconstruction of a single aortic arch branch: a single-centre experience. Interact Cardiovasc Thorac Surg 2017;25:575-81. [Crossref] [PubMed]
- Wang T, Shu C, Li QM, et al. First experience with the double chimney technique in the treatment of aortic arch diseases. J Vasc Surg 2017;66:1018-27. [Crossref] [PubMed]
- Wang T, Shu C, Li M, et al. Thoracic Endovascular Aortic Repair with Single/Double Chimney Technique for Aortic Arch Pathologies. J Endovasc Ther 2017;24:383-93. [Crossref] [PubMed]
- Voskresensky I, Scali ST, Feezor RJ, et al. Outcomes of thoracic endovascular aortic repair using aortic arch chimney stents in high-risk patients. J Vasc Surg 2017;66:9-20.e3. [Crossref] [PubMed]
- Pecoraro F, Lachat M, Cayne NS, et al. Mid-term Results of Chimney and Periscope Grafts in Supra-aortic Branches in High Risk Patients. Eur J Vasc Endovasc Surg 2017;54:295-302. [Crossref] [PubMed]
- Liang WT, Wang S, Zhou J, et al. Total Endovascular Repair of Post-dissection Aortic Arch Aneurysm With Chimney Technique. Ann Thorac Surg 2017;103:e241-3. [Crossref] [PubMed]
- Andrási TB, Grossmann M, Zenker D, et al. Supra-aortic interventions for endovascular exclusion of the entire aortic arch. J Vasc Surg 2017;66:281-97.e2. [Crossref] [PubMed]
- Bosiers MJ, Donas KP, Mangialardi N, et al. European Multicenter Registry for the Performance of the Chimney/Snorkel Technique in the Treatment of Aortic Arch Pathologic Conditions. Ann Thorac Surg 2016;101:2224-30. [Crossref] [PubMed]
- Zou J, Jiao Y, Zhang X, et al. Early- and Mid-term Results of the Chimney Technique in the Repair of Aortic Arch Pathologies. Cardiovasc Intervent Radiol 2016;39:1550-6. [Crossref] [PubMed]
- Mangialardi N, Serrao E, Kasemi H, et al. Chimney technique for aortic arch pathologies: an 11-year single-center experience. J Endovasc Ther 2014;21:312. [Crossref] [PubMed]
- Zhu Y, Guo W, Liu X, et al. The single-centre experience of the supra-arch chimney technique in endovascular repair of type B aortic dissections. Eur J Vasc Endovasc Surg 2013;45:633. [Crossref] [PubMed]
- Feng R, Zhao Z, Bao J, et al. Double-chimney technology for treating secondary type I endoleak after endovascular repair for complicated thoracic aortic dissection. J Vasc Surg 2011;54:212-5. [Crossref] [PubMed]
- Sugiura K, Sonesson B, Akesson M, et al. The applicability of chimney grafts in the aortic arch. J Cardiovasc Surg (Torino) 2009;50:475-81. [PubMed]
- Criado FJ. A percutaneous technique for preservation of arch branch patency during thoracic endovascular aortic repair (TEVAR): retrograde catheterization and stenting. J Endovasc Ther 2007;14:54-8. [Crossref] [PubMed]
- Ohrlander T, Sonesson B, Ivancev K, et al. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther 2008;15:427-32. [Crossref] [PubMed]
- Malkawi AH, Hinchliffe RJ, Yates M, et al. Morphology of aortic arch pathology: implications for endovascular repair. J Endovasc Ther 2010;17:474-9. [Crossref] [PubMed]
- Baldwin ZK, Chuter TA, Hiramoto JS, et al. Double-barrel technique for endovascular exclusion of an aortic arch aneurysm without sternotomy. J Endovasc Ther 2008;15:161. [Crossref] [PubMed]
- Shahverdyan R, Gawenda M, Brunkwall J. Triple-barrel Graft as a Novel Strategy to Preserve Supra-aortic;Branches in Arch-TEVAR Procedures: Clinical Study and Systematic Review. Eur J Vasc Endovasc Surg 2013;45:28-35. [Crossref] [PubMed]
- Lachat M, Veith FJ, Pfammatter T, et al. Chimney and periscope grafts observed over 2 years after their use to revascularize 169 renovisceral branches in 77 patients with complex aortic aneurysms. J Endovasc Ther 2013;20:597. [Crossref] [PubMed]
- Donas KP, Criado FJ, Torsello G, et al. Classification of Chimney EVAR-Related Endoleaks: Insights From the PERICLES Registry. J Endovasc Ther 2017;24:72-4. [Crossref] [PubMed]
- Shahverdyan R, Gawenda M, Brunkwall J. Triple-barrel graft as a novel strategy to preserve supra-aortic branches in arch-TEVAR procedures: clinical study and systematic review. Eur J Vasc Endovasc Surg 2013;45:28-35. [Crossref] [PubMed]
- O'Callaghan A, Mastracci TM, Greenberg RK, et al. Outcomes for supra-aortic branch vessel stenting in the treatment of thoracic aortic disease. J Vasc Surg 2014;60:914-20. [Crossref] [PubMed]
- Dong ZH, Fu WG, Wang YQ, et al. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation 2009;119:735-41. [Crossref] [PubMed]
- Criado FJ, Clark NS, Barnatan MF. Stent graft repair in the aortic arch and descending thoracic aorta: a 4-year experience. J Vasc Surg 2002;36:1121-8. [Crossref] [PubMed]
- Yang J, Xiong J, Liu X, et al. Endovascular chimney technique of aortic arch pathologies: a systematic review. Ann Vasc Surg 2012;26:1014-21. [Crossref] [PubMed]


