
Postoperative management for non-small cell lung cancer harboring EGFR mutations
The discovery of activating mutations in the epidermal growth factor receptor (EGFR) gene has become a “game-changer” in the treatment of non-small cell lung cancer (NSCLC) (1,2). Several randomized controlled studies (RCTs) showed that tyrosine kinase inhibitors (TKIs) of EGFR provided a superior survival benefit over platinum-based chemotherapy for advanced NSCLC harboring activating EGFR-mutations such as deletions in the exon 19 (Del19) and a point mutation in the exon 21 (L858R). Today, systemic treatment with an EGFR-TKI has become a standard treatment of care for advanced EGFR-mutated NSCLC (3). In addition, routine EGFR-testing is recommended in daily clinical practice before starting first-line systemic treatment for patients with advanced non-squamous NSCLC, as activating EGFR-mutations are frequently found in non-squamous NSCLC.
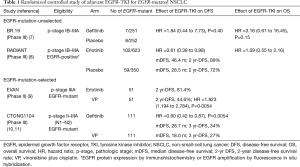
For patients with early-stage NSCLC, surgery is the optimal treatment for the cure. After complete resection, adjuvant platinum-doublet chemotherapy such as vinorelbine plus cisplatin (VP) is recommended for pathologic stage (p-stage) II-III patients based on accumulating clinical evidence shown in several RCTs. However, postoperative adjuvant platinum-doublet chemotherapy has provided only a modest survival benefit of 5–10% improvement in 5-year overall survival rate (4-6). Here, the most important clinical question is whether adjuvant treatment with an EGFR-TKI may provide a superior clinical benefit over that with platinum-doublet chemotherapy for completely resected EGFR-mutated NSCLC. In other words, even for EGFR-mutated patients, platinum-doublet chemotherapy remains the recommended regimen in postoperative adjuvant setting, or systemic treatment with an EGFR-TKI may replace it? To address the question, several RCTs of adjuvant EGFR-TKI treatment have been conducted (Table 1). In an early study (BR.19), all patients with completely resected p-stage IB-IIIA NSCLC were eligible regardless of EGFR-status, and a total of 503 patients were randomly assigned to receive a first-generation EGFR-TKI (gefitinib) or placebo for 2 years (7). Exploratory analyses of only 15 patients with EGFR-mutations demonstrated no survival benefit from gefitinib [hazard ratio (HR), 1.84 for disease-free survival (DFS) and 3.16 for overall survival (OS)]. In another early study (RADIANT), p-stage IB-IIIA NSCLC patients either with EGFR-protein expression-positive by immunohistochemistry or with EGFR-gene amplification-positive by fluorescence in situ hybridization were eligible (8). Patients were randomly assigned to receive another first-generation EGFR-TKI (erlotinib) or placebo for 2 years. Among 161 patients with EGFR-mutations, DFS seemed in favor of the erlotinib group whereas the DFS benefit was not statistically significant.
Full table
In recent randomized studies reported from China (9-12), only EGFR-mutated patients were enrolled, and were assigned to receive a first-generation EGFR-TKI or chemotherapy (VP) (Table 1). The EVAN study is a randomized phase II study conducted for p-stage IIIA NSCLC harboring EGFR-mutations, and the primary endpoint was DFS at 2 years. At the median follow-up of 33 months, the 2-year DFS rate was higher in the erlotinib group (81.4%) than in the chemotherapy group (44.6%; P=0.0054) (9). The CTON1104 (ADJUVANT) study is a formal phase III study to compare the efficacy of adjuvant gefitinib treatment with that of adjuvant chemotherapy with VP. A total of 222 patients with completely resected p-stage II-IIIA (N1-2) NSCLC harboring EGFR-mutations were randomized. The primary endpoint of DFS was significantly longer in the gefitinib group (median DFS, 28.7 months) than in the VP group (18.0 months; P=0.0054) (10). Based on these results, Liang and coworkers have published the Society for Translational Medicine consensus on postoperative management of EGFR-mutant lung cancer (2019 edition) to support postoperative adjuvant treatment with an EGFR-TKI for completely resected p-stage II-IIIA NSCLC with activating EGFR-mutations as well as routine EGFR-testing after surgery for NSCLC (13).
I strongly make an objection against the recommendations. The goal of adjuvant treatment for resected NSCLC patients is to increase the proportion of patient with “cure”, whereas the goal of systemic treatment for advanced un-resectable NSCLC is prolongation of overall survival time. To justify the use of an EGFR-TKI in postoperative adjuvant setting, a significant increase in the proportion of cured patients or those who survived 5 year or longer should be demonstrated in a randomized phase III study. The CTONG1104 study is the only phase III study showing a significant DFS benefit with adjuvant EGFR-TKI treatment for completely resected EGFR-mutated NSCLC. However, in a post hoc analysis of the study, postoperative recurrence was lower in the gefitinib group than in the VP group during early postoperative period (0–21 months after surgery), but recurrence in the gefitinib group has constantly increased at a constant rate 12 months post-surgery (11). These results may indicate that adjuvant treatment with EGFR-TKI do not improve the proportion of cured patients but only delay development of tumor recurrence. For advanced EGFR-mutated NSCLC, systemic treatment with an EGFR-TKI may provide a significant survival benefit, but may not lead to cure in most patients. In postoperative adjuvant setting, elimination of all tumor cells in minimal residual tumor (MRD) may not be achieved with an EGFR-TKI, which is essential to increase the proportion of cured patients. In addition, an EGFR-TKI is active not only for advanced unresectable EGFR-mutated NSCLC, but also for tumor with postoperative recurrence. In fact, the WJTOG3405 study comparing first-line treatment with gefitinib versus chemotherapy [cisplatin plus docetaxel (DP)], a subset analysis showed that the progression-free survival (PFS) was longer in the gefitinib group (13.7 versus 8.1 months) among patients with postoperative recurrence (14), suggesting that EGFR-mutated patients who underwent complete resection were effectively treated with an EGFR-TKI at the time of tumor recurrence. In addition, adjuvant EGFR-TKI treatment may cause postoperative recurrence with resistance to an EGFR-TKI. In advanced NSCLC with activating EGFR-mutations, first-line treatment with a first-generation EGFR-TKI usually achieves a significant tumor shrinkage, but most patients experience tumor progression through development of resistant tumor caused by resistant EGFR-mutations such as T790M and other mechanisms within one year after the initiation of treatment (15). Osimertinib, a third-generation EGFR-TKI can overcome the T790M resistance (16,17), but may induce a variety of complicated resistance mechanisms such as activation of bypass signaling pathways and transformation to small cell carcinoma (18,19). When EGFR-TKI-resistant postoperative tumor recurrence may develop in patients who received adjuvant EGFR-TKI treatment, no effective treatment other than platinum-doublet chemotherapy is currently available. Accordingly, adjuvant treatment with an EGFR-TKI is not recommended for completely resected NSCLC with EGFR-mutations in daily clinical practice, as no RCT showed a significant OS benefit with prophylactic use of an EGFR-TKI before tumor recurrence. I have a concern about on-going large-scale RCTs of adjuvant EGFR-TKI treatment, as most of them was conducted to evaluate DFS as the primary endpoint (Table 2).

Full table
More importantly, a careful attention should be paid to implementation of adjuvant treatment following complete resection, because a certain percentage of patients will be cured without any adjuvant treatment. In fact, RCTs of adjuvant chemotherapy showed that 5-year survival rates for completely resected p-stage II-IIIA NSCLC were 30–50% in the surgery-alone group (4). For such patients who will be cured without adjuvant treatment, adjuvant EGFR-TKI treatment is unnecessary in principle, and is potentially harmful as is associated with several adverse events including lethal interstitial lung disease. To reduce the potential risk of adjuvant EGFR-TKI treatment for potentially curable patients, biomarker-oriented selection of patients who truly need adjuvant treatment due to a higher risk of postoperative recurrence is a promising approach. Among several biomarkers, circulating tumor DNA (ctDNA) is a potentially useful marker not only in predicting postoperative recurrence but also in monitoring therapeutic effect of adjuvant treatment. Today, osimertinib, has become the preferred EGFR-TKI in first-line treatment for advanced EGFR-mutated NSCLC, as is associated with a superior survival benefit (PFS and OS) and toxicity profile over a first-generation EGFR-TKI (gefitinib or erlotinib) (20,21). However, prophylactic use of osimertinib in postoperative adjuvant setting may induce a variety of EGFR-TKI-resistant mechanisms, as is demonstrated in systemic treatment for advanced NSCLC (18,19). The optimal selection of agent in postoperative adjuvant setting as well as the optimal selection of patients may be the key to achieve the optimal risk-benefit balance with adjuvant EGFR-TKI treatment for EGFR-mutated NSCLC patients.
In conclusion, the current clinical evidence may not support the use of adjuvant EGFR-TKI treatment for completely resected EGFR-mutated NSCLC, because only a significant prolongation of DFS after surgery was achieved. On-going RCTs may reveal whether adjuvant EGFR-TKI treatment can improve the postoperative prognosis (Table 2).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-2020-45). FT reports personal fees from AstraZenec, grants and personal fees from Chugai Pharamaceutical, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Eli Lilly and Company, grants and personal fees from Kyowa Kirin Co.Ltd, grants and personal fees from Taiho Pharmaceutical, grants and personal fees from Bristol-Myers Squibb, during the conduct of the study; grants and personal fees from Ono Pharmaceutical, grants and personal fees from MSD Co.Ltd, personal fees from Jhonson & Jhonson Co.Ltd, personal fees from Covidien Japan, outside the submitted work; The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Hanna N, Johnson D, Temin S, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]
- Tanaka F, Yoneda K. Adjuvant therapy following surgery in non-small cell lung cancer (NSCLC). Surg Today 2016;46:25-37. [Crossref] [PubMed]
- NSCLC Meta-analyses Collaborative Group, Arriagada R, Auperin A, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Goss GD, O'Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [Crossref] [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol 2015;33:4007-14. [Crossref] [PubMed]
- Yue D, Xu S, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med 2018;6:863-73. [Crossref] [PubMed]
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. [Crossref] [PubMed]
- Xu ST, Xi JJ, Zhong WZ, et al. The unique spatial-temporal treatment failure patterns of adjuvant gefitinib therapy: a post hoc analysis of the ADJUVANT Trial (CTONG 1104). J Thorac Oncol 2019;14:503-12. [Crossref] [PubMed]
- Cheng H, Li XJ, Wang XJ, et al. A meta-analysis of adjuvant EGFR-TKIs for patients with resected non-small cell lung cancer. Lung Cancer 2019;137:7-13. [Crossref] [PubMed]
- Liang W, Cai K, Chen C, et al. Society for Translational Medicine consensus on postoperative management of EGFR-mutant lung cancer (2019 edition). Transl Lung Cancer Res 2019;8:1163-73. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Wang ZF, Ren SX, Li W, et al. Frequency of the acquired resistant mutation T790 M in non-small cell lung cancer patients with active exon 19Del and exon 21 L858R: a systematic review and metaanalysis. BMC Cancer 2018;18:148. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Oxnard GR, Hu Y, Mileham KF, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol 2018;4:1527-34. [Crossref] [PubMed]
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol 2018;36:841-9. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]


