
Respiratory health effects of residential individual and cumulative risk factors in children living in two cities of the Pearl River Delta Region, China
Introduction
Children spend 80–90% of their time indoors. Due to their developing physiology, children are often more susceptible to indoor pollution (1-3). Indoor pollution sources such as cooking, environmental tobacco smoke (ETS), dampness and molds, chemicals off-gassed from consumer products, may increase the risk for the development of asthma, reduce lung function (4-6), increase airway hyperresponsiveness, and elevate the prevalence and/or incidence of respiratory symptoms in children (4-10). Indoor environment may be associated with many factors potentially interacting with each other and affecting each other. However, previous studies have mainly focused on single exposure factors when exploring the health risks relevant to indoor environment. This is not consistent with the fact that health risks are the results of combined actions of multiple exposure factors.
In an attempt to overcome the existing limitation, we developed a method for integrating multiple indoor exposure factors. On the basis of the random forest algorithm, the method aimed to identify high-priority indoor environmental risk factors and generated a composite index for indoor environmental exposure. The random forest algorithm is a powerful classification and regression approach capable of measuring variable importance and identifying the interaction of variables to enhance the predictive accuracy (11). It has the major advantages of preventing overfitting and producing improved predictive accuracy, therefore has gained considerable popularity in the field of bioinformatics (12-15). The data analysis in our study for identifying the most important indoor health risk factors is inspired by the application of random forest in bioinformatics that has been developed to screen the genes most relevant to diseases.
In this context, we analyzed data collected in a cross-sectional study of 2,306 children in the cites of Guangzhou and Shenzhen, China. The purpose of the present analysis was two-fold. First, we aimed to explore the health effects of individual indoor environmental risk factors. Secondly, we used a random forest algorithm to form a composite risk index integrating most important individual risk factors, aiming at assessing the cumulative risk of indoor pollution (CRIP).
Methods
Study design
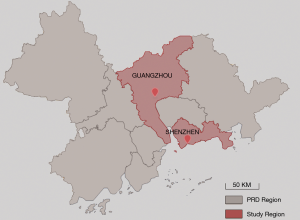
Our study draws from an extended study of the Four Chinese Cities Study (4CC study) which was originally conducted in 1993–1997 (16). The current study conducted in 2018 was a follow-up of the 4CC study to explore the health effects of the changes in environmental risk factors over 20 years (17). Similar to the 4CC study, children were sampled from two elementary schools located in urban and suburban of each city in the current extended study. As part of the larger 4CC study, our study was undertaken in the cities of Guangzhou and Shenzhen. As one of the cities with the fastest economic development in China, Guangzhou has extensively expanded its urban areas in the past 20 years, resulting in high urbanization of suburban areas and spatial homogeneity of air pollution across urban and suburban areas. To better reflect the heterogeneity in environmental pollution effects, the city of Shenzhen, located about 150 km southeast of Guangzhou, was introduced into our current study as a contrast area of Guangzhou (Figure 1). Shenzhen has similar climate to Guangzhou but with generally lower air pollution levels. Both of the cities are within the Pearl River Delta region with warm and humid weather. The mean annual temperature ranges from 14 to 22 °C and annual precipitation is 1,525.1 mm (18). Hence buildings in these cities are highly vulnerable to indoor dampness and mold. Two elementary schools, one located at Huangpu District, Guangzhou, and the other in university town of Nanshan District, Shenzhen, were selected for the study. Both schools were located at the upwind areas of the Pearl River Delta region and less than 100 meters distant from the nearest main road. There are no obvious industrial pollution sources within a radius of 1km from the schools. Children in grades 1 to 6 were all recruited from each school from December, 2017 to May, 2018. We used a unified study protocol for questionnaire survey and lung function measurement. The standardized questionnaires were used to collect data on environmental exposure and respiratory symptoms and diseases. Lung function was measured using the same models of spirometers following the same QA/QC guidelines. This study was approved by the Duke Kunshan University Institutional Review Board (DKU IRB) (No. FWA00021580). Informed consent forms were obtained from parents or guardians of the children before they participated in the study.
Questionnaire survey
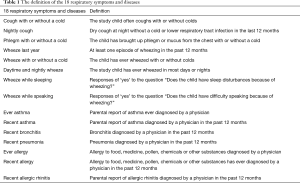
Questionnaires were completed by children’s parents to obtain information on household characteristics (e.g., ETS exposure, stove/fuel type, cooking habit, kitchen type, ventilation pattern, home dampness and molds), children’s respiratory health status, parental information (including health histories, occupation and education). The questionnaire, which had previously been validated in the 4CC study (16), was a modified version of the American Thoracic Society Epidemiologic Standardization Project questionnaire (19). We asked 2,765 families (1,565 in Guangzhou and 1,200 in Shenzhen) to fill out the study questionnaire and received 2,420 questionnaires (response rate =87.5%). After excluding those with missing data, 2306 were included in our analysis. For the present study, we used all the indoor environment variables and respiratory symptoms/diseases variables (Table 1) collected in the questionnaires.
Full table
The indoor environment characteristics (potential risk factors) are defined as following:
Dampness and molds: there were visible molds in the house due to dampness in the past 12 months; ETS: child lived with any family members who were smokers; incense burning: Household burned incense stick or mosquito-repellent incense during summer; open kitchen: child’s residence had an open kitchen; decoration:child residence was decorated (e.g., interior remodeling, new furnishing, and new surface painting) in the past 12 months; cooking frequency: this was classified as “high” if child’s home cooked for more than 3 days a week and “low” if cooked ≤3 days/week; pets: child’s household kept one or more pets at home; air conditioner: child’s household used air conditioning for more than 5 hours a day in any one of the four seasons; kitchen ventilation: household used a mechanical ventilator in the kitchen, including exhaust fan or smoke exhaust ventilator; non-clean fuels: household used gas or solid fuels for cooking (reference is electricity for cooking); air freshener: household used air fresheners at home.
Lung function measurement
Among the children who had complete questionnaire data as described above, 1,044 students aged 5–13 years with the male-to-female ratio of 1:1 in grades 1 to 6 were selected for lung function tests by stratified random sampling. Approximately equal number of students were selected from each grade in each of the two schools. After excluding those with missing data in the questionnaire and with invalid values for their lung function measure, 987 children (484 from Guangzhou and 503 from Shenzhen) were included in the data analysis.
Lung function was measured using a spirometer (Spirolab III, Medical International Research, Rome, Italy) by trained research technicians according to the American Thoracic Society guideline. Children were instructed to perform the lung function test in a standing position wearing a nose clip. The best of three acceptable spirometry maneuvers was selected. The following lung function variables were included in our data analyses: forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), peak expiratory flow (PEF), forced expiratory flow at 25% of expired volume (FEF25%), forced expiratory flow between 25% and 75% of expired volume (FEF25–75%), forced expiratory flow at 75% of expired volume (FEF75%), maximum voluntary ventilation (MVV), and vital capacity (VC).
Statistical analysis
Variable importance ranking by random forest algorithm
We identified high-priority indoor environmental risk factors using machine learning algorithm of random forest. Random forest has prominent performance in classification and regression, and is capable of providing variable importance measures to examine the extent to which each variable contributes to the estimate of magnitude of effect as a part of the results. The variable importance measures based on the random forest are dependent on Mean Decrease Gini; the larger the value of the indicator is, the more important the variable is (11). Our study included 11 indoor environmental risk factors (i.e., home dampness and molds, ETS exposure, incense burning, open kitchen, household decoration, cooking frequency, pets, use of air conditioner, kitchen ventilation, cooking fuels, and use of air freshener) and 18 health outcomes. Based on the Gini index, we evaluated the importance of 11 indoor environmental risk factors and ranked their risks. The 11 indoor variables were ranked in the order of smallest to largest in variable importance measures from each forest (N=18). We then assigned a weight to each indoor variable corresponding to the ranks where a variable appeared. Finally, the total score of variable importance measures for each variable was obtained by summing up its weight within each ranked list. More information is provided in supplementary material (see supplementary and Table S1).

Full table
Association between indoor exposure variables and health outcomes
We used both simple and multiple logistic regression models to analyze the relationship between selected indoor risk factors and respiratory diseases or symptoms. Age, maternal education, breastfeeding duration, maternal smoking during pregnancy, maternal asthma, paternal asthma and other covariates were adjusted in the logistic regression models. When examining the relationship between indoor exposure variables and lung function, we used multivariate linear regression models in which lung function data were natural logarithm transformed and child’s age, sex, height, and weight were included as covariates. The exponentiated values of regression coefficients from the linear regression models represent the percentage changes in lung function associated with the change in an exposure variable from the reference level.
Assessment of CRIP
The results from the simple logistic regression showed that the presence of pets was a protective factor for children’s respiratory health in our study (Table S2). As the purpose of this study was to identify the effect of household environmental risk factors, we excluded the presence of pets; and the first six risk variables with high scores were included in an integrated model. The six variables in the model were home dampness and molds, ETS, incense burning, open kitchen, household decoration, and cooking frequency. We integrated these 6 variables to generate a comprehensive index named the CRIP, which we developed for use in this study to assess the cumulative risk of multiple indoor environmental exposures. The CRIP models were shown in Figure S1A,B. The six risk factors were defined as binary variables. The hierarchical arrangement referred to the ranked list of the six risk factors using variable importance measures from the random forest. If four out of the six risk variables were considered as ‘high risk’, higher CRIP was assigned to the child. Logistic regressions and multivariate linear regressions were used to examine the association of CRIP with respiratory diseases and lung function, respectively.
Full table

The random forest analyses were performed by the random Forest package in R 2.5.3 (version 4.6-14, R Foundation for Statistical Computing, Vienna, Austria). Logistic regression and multiple linear regression models were performed using Stata (version 15.0; Stata Corp LP, College Station, TX, USA).
Results
Description analysis
The characteristics of the study objects and indoor exposure variables are shown in Table 2. Among the 2,306 children, 253 were excluded from analysis due to missing data. More than 50% of mothers had received undergraduate education or higher. The prevalence of maternal asthma and paternal asthma was each below 1.0%. The proportion of maternal smoking during pregnancy was low (0.45%). However, 44.83% of the children were reportedly exposed to ETS, due to paternal, maternal, and other family member’s smoking. Dampness and mold occurred in about a quarter of children’s houses in the last 12 months. The vast majority of households (94.09%) used non-clean (gas) fuels (versus electricity) and 89.50% reported home cooking more than 3 days a week. About half of the children had cough with a cold. The prevalence of nightly cough, wheeze, bronchitis and allergic rhinitis were relatively high in the last 12 months, which was 22.48%, 24.63%, 14.95% and 15.54%, respectively. The prevalence of ever asthma (2.85%) and recent asthma (1.07%) were low. Among all the subjects, 314 (15.29%) had a higher CRIP score.

Full table
Of the 2,306 children, 987 children were measured for their lung function but 73 were excluded due to missing data in analysis of CRIP. We found that 121 (13.24%) of these children lived in homes with a higher CRIP score. The geometric means of lung function parameters are presented in Table 2.
Associations between indoor exposure and respiratory health outcomes
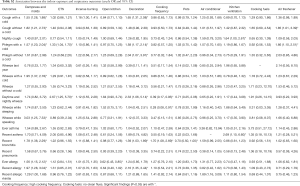
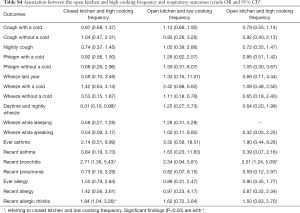
Adjusted odds ratios (Table 3) for home dampness and molds in association with cough with or without a cold, phlegm with a cold, wheeze with a cold, wheeze while speaking, recent bronchitis, ever allergy, recent allergy and recent allergic rhinitis were greater than 1 and statistically significant (P<0.05). Indoor ETS exposure and household decoration in the past year were also associated with a variety of respiratory diseases and symptoms. Children exposed to ETS were 1.37 times more likely to develop phlegm with a cold, 2.94 times more likely for daytime and nightly wheeze, 4.09 times more likely for wheeze during last year, and 3.58 times more likely for wheeze while sleeping. Children who had household decoration in the previous year were more susceptible to cough with a cold, wheeze while sleeping and recent allergy than those without home decoration. The ORs were >1 and statistically significant (P<0.05). The results of unadjusted ORs are provided in Table S2. The results were similar between unadjusted and adjusted ORs. In the adjusted model, children living in houses with open kitchens were 3.93 times more likely to be at risk for wheeze while sleeping than those living in houses with closed kitchens. It was noted that an open kitchen was a protective factor for ever allergy (OR =0.62, 95% CI: 0.45–0.85), and high cooking frequency was negatively associated with daytime and nightly wheeze and wheeze while sleeping (P<0.05). To evaluate the combined health effects of kitchen type and cooking frequency to avoid potential confounding, we conducted a sensitivity analysis (Table S3). Children living in residences with an open kitchen and with a high cooking frequency were more likely to develop bronchitis than those living residences with a closed kitchen and a low cooking frequency (OR =2.51, 95% CI: 1.24–5.09, Table S4).
Full table

Full table
Full table
Association between indoor exposure and lung function
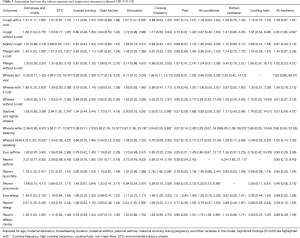
The relationships between indoor exposure variables and lung function are shown in Table 4. After adjusting for physiological factors (age, gender, height and weight), home dampness and molds were negatively associated with FEF25–75% (P=0.047) and FEF75% (P=0.037). There were significantly negative associations between ETS exposure and VC (P=0.022), mechanical kitchen ventilation and PEF (P=0.041), use of air freshener and FEF25% (P=0.032). We found statistically significant associations between open kitchens and reductions in PEF (P=0.025), VC (P=0.003) and FEF25% (P=0.044), respectively. We also found a statistically significant association between incense burning and reduced VC (P=0.032). However, most of the remaining indoor risk factors showed non-significant associations with children’s lung function. We also noted kitchen ventilation was significantly and negatively associated with PEF, and theorized that this unexpected association might result from bias due to without considering cooking frequency in the model. A sensitivity analysis was conducted to evaluate the combined effects of kitchen ventilation and cooking frequency to avoid potential confounding (Tables S5,S6). These subgroup models showed that children living in residences without kitchen ventilation, whether with low or high cooking frequency, were more likely to have lower lung function, compared to those living in residences with kitchen ventilation.

Full table

Full table

Full table
After adjusting for age, gender, height, weight, maternal education, breastfeeding duration, and other indoor factors in the model (Table S7), we only found significant associations of open kitchen with reduced VC (P=0.003), and the use of air freshener with reduced PEF (P=0.038), reduced FEF25–75% (P=0.044) and reduced FEF25% (P=0.012), respectively. Other indoor risk factors showed negative but nonsignificant associations with the lung function parameters. Findings of the present study provide robust evidence that lung function, as measured by PEF, FEF25–75% and FEF25%, is reduced in children exposed to indoor air freshener. This result is expected because air freshener is an important source of volatile organic compounds (VOCs), most of which have been found to cause adverse respiratory effects (20-22). It was noted, however, that the use frequency and duration of air freshener were not investigated in the current study; thus, the findings should be interpreted with caution.

Full table
Association between the CRIP and respiratory diseases and symptoms
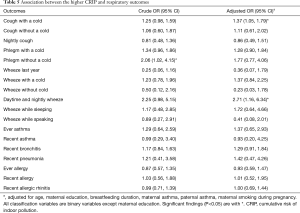
We evaluated the CRIP based on the six risk variables (including dampness and molds, ETS exposure, incense burning, open kitchen, household decoration and cooking frequency). As shown in Table 5, children with a higher CRIP score were more likely to have phlegm without a cold (OR =2.06, 95% CI: 1.02–4.15) than those with a lower CRIP score. As for other respiratory diseases or symptoms, the results indicated that children with a higher CRIP score also had a higher risk.
Full table
After the adjustment for age, maternal education, breastfeeding duration, maternal smoking during pregnancy, maternal asthma and paternal asthma, children with a higher CRIP score were 1.37 times (95% CI: 1.05–1.79) more likely to have cough with a cold and 2.71 times (95% CI: 1.16–6.34) more likely to develop daytime and nightly wheeze.
Association between the CRIP and lung function
As shown in Table 6, after adjusting for age, gender, height and weight in the multivariate linear regression models, we found that higher CRIP scores were significantly associated with lower VC values (−6.42%, P=0.034). CRIP scores were negatively (without statistical significance) associated with most of the other lung function variables. After further adjusting for maternal education, breastfeeding duration, and maternal smoking during pregnancy, the findings remain unchanged.

Full table
Discussion
Our study presents an approach that can evaluate the CRIP. Home dampness and molds, ETS exposure, incense burning, open kitchen, household decoration and cooking frequency are among the most important predictor variables for children’s indoor exposure risks. We found significant effects of home dampness and molds as well as ETS exposure on children’s respiratory symptoms and lung function measures. Children living in houses with a higher CRIP score were more likely to report respiratory symptoms and to have reduced lung function.
Previous studies have found associations of home dampness and molds with respiratory diseases and symptoms in children (23-25). Our findings on increased risks of respiratory diseases (i.e., cough with a cold, cough without a cold, wheeze while speaking, recent bronchitis, recent allergy, recent allergic rhinitis) in children living in homes with dampness and molds are consistent with the finding from a birth cohort study of 4,098 children in Sweden. In this 16-year-follow-up study, the presence of home dampness and molds was associated with an increased risk of asthma (OR =1.31; 95% CI: 1.08–1.59) and rhinitis (OR =1.28; 95% CI: 1.04–1.58) in children (26). Home dampness exposure in our study was associated with not only increased risk of respiratory symptoms but also reduction in FEF25–75% and FEF75% (Table 4). Most previous studies showed consistent, albeit heterogeneous, negative association of home dampness with acute changes in lung function (5,27). Plausible mechanisms of home dampness and molds adverse effects have been well described (26,28,29). Childhood exposure to dampness and molds may induce respiratory irritation and activate immune system, resulting in chronic respiratory inflammation and other inflammatory diseases such as rhinitis. Bioaerosols (e.g., fungal spore) were also suggested to contribute to the adverse health effects of home dampness as well (30).
A large number of harmful substances in ETS have been confirmed to trigger toxic injury to mucous epithelium and immunocytes, causing long-term inflammation and hyperemia of respiratory airway (31-34), and increasing the ability of cell adherence of microorganism to respiratory epithelial (35,36). These potential pathophysiologic pathways support ETS exposure as a risk factor for respiratory symptoms including bronchitis and wheeze. Our results further demonstrate the harmful effects of ETS exposure on the respiratory health, reflected in increased risks for phlegm with a cold, wheeze last year, daytime and nightly wheeze and wheeze while sleeping. Our finding is consistent with the results of the previous studies (10,23,37-39).
Although the use of household solid fuel has been considered to be the major source of indoor pollution, few of our subjects’ households used solid fuels. The majority used gas fuels. We did not find a significant effect of gas fuel use in the present study. A study among 2,289 United Kingdom subjects found that gas cooking (compared to electricity cooking) was significantly associated with increased odds of wheeze in children (OR =1.47; 95% CI: 1.05–1.74) (37). However, a Dutch birth cohort of more than 3,000 children only found a significant association of gas cooking with nasal symptoms, but not with other respiratory diseases or allergic diseases (40). An Australian study including 2,815 participants suggested that gas cooking was slightly associated with lung function reduction in children (41). Considerable inconsistencies among the findings of different studies could be attributable to heterogeneity in the effects of household characteristics and exposure assessment approaches (42).
The random forest model described here is a useful method for variable selection. It allowed for the identification of household risk factors that were associated with children’s respiratory diseases or symptoms, and even potential risk factors that were not of concerns in previous studies. It also allowed for estimating variable importance and predicting risk ranking of household environmental risk factors for our study. Based on the random forest algorithm, we identified that dampness and molds, ETS exposure, use of mosquito-repellent incense, open kitchen, household decoration and cooking frequency were top-ranked in terms of variable importance among the indoor environment risk factors. This resulted in the development of the CRIP index. Adjusted for age, maternal education and breastfeeding duration, maternal smoking during pregnancy and other covariates, children with higher CRIP was positively associated with the risks of cough with a cold (OR =1.37; 95% CI: 1.05–1.79) and daytime and nightly wheeze (OR=2.71; 95% CI: 1.16–6.34). And children with a higher CRIP score was negatively associated with FVC, FEV1, PEF, FEF25–75%, VC and FEF25%, but statistically significant association was found only for VC (P=0.029). Taking combined action of multiple exposure factors into account, our CRIP model provided a comprehensive reflection of the health effects of indoor exposure for children. The CRIP index is straightforward and simple for identifying importance and effects of environmental risk factors. It can be applied to, but not limited to indoor environment, any environmental media to estimate combined effects of multiple risk factors. Our study demonstrates the usefulness of using the random forest data analytic approach in the health risk assessment. Considering the “explosion” in our data collection capacity and the rapid advancement in data science, the application of big data analysis (e.g., machine learning, and deep learning) in environmental health research holds great promise to address multiple risk factors.
There are limitations in our study. The information about indoor exposure for the CRIP index was based on parental-reported questionnaire. The socioeconomic status and outdoor environmental factors of each study subject were not considered or adjusted in the CRIP index due to the lack of data. The cross-sectional study design has its inherent limitations of potential confounding. Finally, data on respiratory symptoms and illnesses were derived from self-reporting via a questionnaire survey, which has potential recall and reporting biases.
Conclusions
Exposure to home dampness and molds was a risk factor for respiratory health in school children living in Guangzhou and Shenzhen, located in a subtropical region. Given that these southern China coastal cities have many months of high-humidity weather conditions, moisture control is an important preventive measure to reduce children’s respiratory symptoms. A Random-Forest based method was useful to generate a CRIP that represents the combined effects of multiple risk factors. Higher CRIP values were associated with increased respiratory symptoms and reduced lung function.
Supplementary
Statistical analysis: variable important measures by random forest
The random forest algorithm is a powerful classification and regression approach capable of measuring variable importance and identifying the interaction of variables to enhance the predictive accuracy. The algorithm operates by extracting several subsamples, forming bootstrap training sets, generating a large number of decision trees, and letting these classifiers “vote” to form the final predictor (11).
Our study included 11 indoor environmental risk factors (i.e., home dampness and molds, ETS exposure, incense burning, open kitchen, household decoration, cooking frequency, pets, use of air conditioner, kitchen ventilation, cooking fuels, and use of air freshener) and 18 health outcomes (cough with a cold, cough without a cold, nightly cough, phlegm with a cold, phlegm without a cold, wheeze last year, wheeze with a cold, wheeze without a cold, daytime and nightly wheeze, wheeze while sleeping, wheeze while speaking, ever asthma, recent asthma, Recent bronchitis, recent pneumonia, ever allergy, recent allergy, recent allergic rhinitis). To perform the random forest analyses, first, several (N=500) bootstrap samples were randomly drawn from the original data as the training set data. The training sets were used to establish unpruned classification trees with square root of M (M=11, representing 11 indoor environmental risk factors) predictors randomly sampled. The remaining 1/3 out-of-bag (OOB) samples, which were not included in the bootstrap samples, were used for cross-validation. Finally, based on the Gini Index, we evaluated the importance of predictors and ranked the risk of 11 indoor environmental risk factors.
The 11 indoor variables were ranked in the order of smallest to largest in variable importance measures from each forest (N=18). We then assigned a weight to each indoor variable corresponding to the ranks where a variable appeared. Finally, the total score of variable importance measures for each variable was obtained by summing up its weight within each ranked list (Table S1).
Five random forest models where the OOB error were more than 20% were excluded, including the forest with the dependent variables of cough with a cold, night cough, wheeze, wheeze while sleeping and wheeze while speaking. The total scores of variable importance measures for each variable of the rest 13 models was also provided in the Table S1. The orders of variable importance were similar whether the 5 forests were excluded or not.
Acknowledgments
We thank the participants of the two elementary schools in our study for their diligent efforts. We greatly appreciate Dr. Qin Liu (ChongQing Medical University) for her great help in comments and suggestions for the manuscript. We are grateful to Dr. Yajun Chen (Department of Maternal and Child Health, School of Public Health, Sun Yat-sen University) and Ms. Lu Deng (Center for Health Promotion for Primary and Secondary Schools, Guangzhou Education Bureau) for the assistance in recruitment by the elementary school in Guangzhou. We thank the volunteers in our group for all the support in the sampling and quality assurance of the study.
Funding: This work was supported by the National Natural Science Foundation of China (grant numbers 41501540 and 91743109) and the Guangdong Province Natural Science Foundation (grant number 2016A030313194).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Junfeng Zhang, Howard Kipen and Haidong Kan) for the focused issue “Children’s Respiratory Health and Air Quality” published in Journal of Thoracic Disease. The article was sent for peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.92). The issue “Children’s Respiratory Health and Air Quality” was commissioned by the editorial office without any funding or sponsorship. JJZ served as the unpaid Guest Editor of the issue. JJZ also serves as an unpaid editorial board member of Journal of Thoracic Disease. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Duke Kunshan University Institutional Review Board (DKU IRB) (No. FWA00021580), and parents or other guardians of the children signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Klepeis NE, Nelson WC, Ott WR, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol 2001;11:231-52. [Crossref] [PubMed]
- Stranger M, Potgieter-Vermaak SS, Van Grieken R. Characterization of indoor air quality in primary schools in Antwerp, Belgium. Indoor Air 2008;18:454-63. [Crossref] [PubMed]
- Canha N, Freitas MC, Almeida SM, et al. Indoor school environment: easy and low cost to assess inorganic pollutants. J Radioanal Nucl Chem 2010;286:495-500. [Crossref]
- Lannerö E, Wickman M, van Hage M, et al. Exposure to environmental tobacco smoke and sensitisation in children. Thorax 2008;63:172-6. [Crossref] [PubMed]
- Holst GJ, Host A, Doekes G, et al. Allergy and respiratory health effects of dampness and dampness-related agents in schools and homes: a cross-sectional study in Danish pupils. Indoor Air 2016;26:880-91. [Crossref] [PubMed]
- Chao MR, Cooke MS, Kuo CY, et al. Children are particularly vulnerable to environmental tobacco smoke exposure: Evidence from biomarkers of tobacco-specific nitrosamines, and oxidative stress. Environ Int 2018;120:238-45. [Crossref] [PubMed]
- Dales R, Liu L, Wheeler AJ, et al. Quality of indoor residential air and health. CMAJ 2008;179:147-52. [Crossref] [PubMed]
- Lin W, Brunekreef B, Gehring U. Meta-analysis of the effects of indoor nitrogen dioxide and gas cooking on asthma and wheeze in children. Int J Epidemiol 2013;42:1724-37. [Crossref] [PubMed]
- Vesper S, Wymer L. The relationship between environmental relative moldiness index values and asthma. Int J Hyg Environ Health 2016;219:233-8. [Crossref] [PubMed]
- Olaniyan T, Dalvie MA, Roosli M, et al. Asthma-related outcomes associated with indoor air pollutants among schoolchildren from four informal settlements in two municipalities in the Western Cape Province of South Africa. Indoor Air 2019;29:89-100. [Crossref] [PubMed]
- Breiman L. Random forests. Mach Learn 2001;45:5-32. [Crossref]
- Lunetta KL, Hayward LB, Segal J, et al. Screening large-scale association study data: exploiting interactions using random forests. BMC Genet 2004;5:32. [Crossref] [PubMed]
- Hoffmann K, Firth MJ, Beesley AH, et al. Translating microarray data for diagnostic testing in childhood leukaemia. BMC Cancer 2006;6:229. [Crossref] [PubMed]
- Chen X, Liu CT, Zhang M, et al. A forest-based approach to identifying gene and gene gene interactions. Proc Natl Acad Sci U S A 2007;104:19199-203. [Crossref] [PubMed]
- Chen X, Ishwaran H. Random forests for genomic data analysis. Genomics 2012;99:323-9. [Crossref] [PubMed]
- Zhang JJ, Hu W, Wei F, et al. Children’s Respiratory Morbidity Prevalence in Relation to Air Pollution in Four Chinese Cities. Environ Health Perspect 2002;110:961-7. [Crossref] [PubMed]
- Yin Z, Huang X, He L, et al. Trends in ambient air pollution levels and PM2.5 chemical compositions in four Chinese cities from 1995 to 2017. J Thorac Dis 2020;12:6396-410.
- Zhang Q, Xiao MZ, Singh VP, et al. Regionalization and spatial changing properties of droughts across the Pearl River basin, China. Journal of Hydrology 2012;472-473:355-66. [Crossref]
- Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 1978;118:1-120. [PubMed]
- Kim S, Hong SH, Bong CK, et al. Characterization of air freshener emission: the potential health effects. J Toxicol Sci 2015;40:535-50. [Crossref] [PubMed]
- Elliott L, Longnecker MP, Kissling GE, et al. Volatile organic compounds and pulmonary function in the Third National Health and Nutrition Examination Survey, 1988-1994. Environ Health Perspect 2006;114:1210-4. [Crossref] [PubMed]
- Anderson RC, Anderson JH. Toxic effects of air freshener emissions. Arch Environ Health 1997;52:433-41. [Crossref] [PubMed]
- Mommers M, Jongmans-Liedekerken AW, Derkx R, et al. Indoor environment and respiratory symptoms in children living in the Dutch-German borderland. Int J Hyg Environ Health 2005;208:373-81. [Crossref] [PubMed]
- Mendell MJ, Mirer AG, Cheung K, et al. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect 2011;119:748-56. [Crossref] [PubMed]
- Zacharasiewicz A, Zidek T, Haidinger G, et al. Symptoms suggestive of atopic rhinitis in children aged 6-9 years and the indoor environment. Allergy 2000;55:945-50. [Crossref] [PubMed]
- Thacher JD, Gruzieva O, Pershagen G, et al. Mold and dampness exposure and allergic outcomes from birth to adolescence: data from the BAMSE cohort. Allergy 2017;72:967-74. [Crossref] [PubMed]
- Simoni M, Cai GH, Norback D, et al. Total viable molds and fungal DNA in classrooms and association with respiratory health and pulmonary function of European schoolchildren. Pediatr Allergy Immunol 2011;22:843-52. [Crossref] [PubMed]
- Fisk WJ, Lei-Gomez Q, Mendell MJ. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air 2007;17:284-96. [Crossref] [PubMed]
- Karvonen AM, Hyvarinen A, Korppi M, et al. Moisture damage and asthma: a birth cohort study. Pediatrics 2015;135:e598-606. [Crossref] [PubMed]
- Tischer CG, Heinrich J. Exposure assessment of residential mould, fungi and microbial components in relation to children's health: achievements and challenges. Int J Hyg Environ Health 2013;216:109-14. [Crossref] [PubMed]
- Matulionis DH. Effects of cigarette smoke generated by different smoking machines on pulmonary macrophages of mice and rats. J Anal Toxicol 1984;8:187-91. [Crossref] [PubMed]
- Fukuma M, Seto Y, Fukushima K, et al. The effect of food dye and other environmental substances on the host defense reaction in mice in relation to virus infection. J Toxicol Sci 1986;11:169-77. [Crossref] [PubMed]
- Nair MP, Kronfol ZA, Schwartz SA. Effects of alcohol and nicotine on cytotoxic functions of human lymphocytes. Clin Immunol Immunopathol 1990;54:395-409. [Crossref] [PubMed]
- Hasséus B, Wallstrom M, Osterdahl BG, et al. Immunotoxic effects of smokeless tobacco on the accessory cell function of rat oral epithelium. Eur J Oral Sci 1997;105:45-51. [Crossref] [PubMed]
- Fainstein V. M.Musher D. Bacterial Adherence to Pharyngeal Cells in Smokers, Nonsmokers, and Chronic Bronchitics. Infect Immun 1979;26:178-82. [Crossref] [PubMed]
- Raman AS, Swinburne AJ, Fedullo AJ. Pneumococcal adherence to the buccal epithelial cells of cigarette smokers. Chest 1983;83:23-7. [Crossref] [PubMed]
- de Bilderling G, Chauhan AJ, Jeffs JA, et al. Gas cooking and smoking habits and the risk of childhood and adolescent wheeze. Am J Epidemiol 2005;162:513-22. [Crossref] [PubMed]
- He QQ, Wong TW, Du L, et al. Environmental tobacco smoke exposure and Chinese schoolchildren's respiratory health: a prospective cohort study. Am J Prev Med 2011;41:487-93. [Crossref] [PubMed]
- Liu MM, Wang D, Zhao Y, et al. Effects of outdoor and indoor air pollution on respiratory health of Chinese children from 50 kindergartens. J Epidemiol 2013;23:280-7. [Crossref] [PubMed]
- Willers SM, Brunekreef B, Oldenwening M, et al. Gas cooking, kitchen ventilation, and asthma, allergic symptoms and sensitization in young children--the PIAMA study. Allergy 2006;61:563-8. [Crossref] [PubMed]
- Moshammer H, Fletcher T, Heinrich J, et al. Gas cooking is associated with small reductions in lung function in children. Eur Respir J 2010;36:249-54. [Crossref] [PubMed]
- Dong GH, Qian Z, Fu Q, et al. A Multiple Indicators Multiple Cause (MIMIC) model of respiratory health and household factors in Chinese children: the seven Northeastern cities (SNEC) study. Matern Child Health J 2014;18:129-37. [Crossref] [PubMed]


