Clinical characteristics and survival in idiopathic pulmonary fibrosis and connective tissue disease-associated usual interstitial pneumonia
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrosing interstitial pneumonia of unknown cause characterized by a histopathological and/or radiological pattern of usual interstitial pneumonia (UIP) (1). IPF primarily occurs in older adults and has a median survival rate that varies between 2.5 and 3.5 years from the time of diagnosis (1).
UIP is frequently observed in patients with a variety of systemic connective tissue diseases (CTD). Moreover, CTD-associated UIP (CTD-UIP) shares similar physiological aberrations, pathological patterns and radiological severity with IPF/UIP. Previous analyses of differences in the survival rates of patients with CTD-associated lung fibrosis and IPF/UIP have yielded inconsistent results. Some studies suggest that patients with CTD-associated lung fibrosis have a better prognosis compared with IPF patients (2-4). However, other studies have reported that patients with CTD and concurrent lung fibrosis did not exhibit an improved survival (5-7).
Based on these data, we investigated the differences in the clinical characteristics and prognoses of patients diagnosed with CTD-UIP and IPF/UIP.
Methods
Study population
Patient selection
A retrospective review of the database at the tertiary referral Interstitial Lung Disease (ILD) center at King Khalid University Hospital was completed. Consecutive patients who were newly diagnosed with IPF and CTD-UIP between January 2008 and June 2013 were included. The exclusion criteria included idiopathic interstitial pneumonias other than IPF, chronic hypersensitivity pneumonitis, nonspecific interstitial pneumonia, and a diagnosis of drug-induced or unclassified pulmonary fibrosis.
Patient assessment
A standard form was used to collect clinical information, including general symptoms, systemic symptoms associated with autoimmune diseases proposed by Kinder and colleagues (8) (including Raynaud’s phenomenon, arthralgia/multiple joint swelling, photosensitivity, unintentional weight loss, morning stiffness, dry mouth or eyes (sicca features), dysphagia, recurrent unexplained fever, gastroesophageal reflux, skin rash, oral ulceration, nonandrogenic alopecia and proximal muscle weakness), smoking history, medication use, and physical exam findings. IPF was diagnosed according to established guidelines (1,9). All IPF cases diagnosed prior to the year 2011 were revaluated to ensure that the diagnosis adhered to the current international guidelines on the diagnosis and management of IPF (1). CTD was diagnosed if the patients fulfilled the established classification criteria for rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis (scleroderma), polymyositis/dermatomyositis, Sjogren’s syndrome, or mixed CTD (10-15). The patients were diagnosed with undifferentiated CTD (UCTD) if they exhibited two or more symptoms and signs of autoimmune disease suggestive of CTD and a positive autoimmune serology without fulfilling the well-recognized classification criteria for a CTD (16,17). All CTD patients were observed regularly by rheumatologists. A pulmonary function test (PFT), arterial blood gas (ABG) measurement, six-minute walk test (6MWT), serological tests, and high-resolution computed tomography (HRCT) imaging were performed within 3 days of the initial evaluation if ILD was suspected.
A surgical lung biopsy was performed in 24 (27%) patients with IPF and 15 (22%) patients with CTD-UIP (including ten UCTD patients) because their HRCT images were categorized as atypical for UIP (i.e., their HRCT scans lacked honeycombing and/or displayed predominantly ground glass opacities). A multidisciplinary approach that involved various specialties, including pulmonology, rheumatology, radiology and pathology, was implemented for all ILD patients before a final diagnosis was rendered. The follow-up period for our analysis of survival data ended in June 2014.
This study was approved by the Institutional Review Board and Ethics Committee of the College of Medicine, King Saud University, Riyadh, Saudi Arabia. Written informed consent was waived because of the retrospective review of the database.
Measurements
Spirometry, plethysmography, and the diffusion capacity of the lung for carbon monoxide (DLco) (PFT Masterscreen; Jaeger, Hoechberg, Germany) were measured using established standard methodologies (18-20). ABG values (Rapid Lab 865; Bayer, Plymouth, UK) were used to determine the partial pressure of oxygen (PaO2), the partial pressure of carbon dioxide (PaCO2), and the oxygen saturation (SaO2). After the PFT and ABG sampling, the patients were asked to perform a 6MWT in accordance with ATS guidelines (21). Oxygen saturation by pulse oximetry (SpO2) was recorded at the beginning (initial SpO2) and end (final SpO2) of the six-minute walk. At the end of the test, the total distance walked in meters was documented.
High-resolution computed tomography (HRCT)
All patients underwent computed tomography scanning (Light Speed 16 or VCT XT; GE Medical Systems, Milwaukee, WI, USA). Full-volume scans reconstructed every 2.5 mm were obtained throughout the entire thorax. The scans were performed during suspended inspiration with the patients in a supine position. Additional limited scans that used 1.25-mm thin collimation at 10-mm intervals from the aortic arch level to the lung bases were obtained with high spatial resolution reconstruction during end-expiration with the patients in a prone position.
Serological data
The levels of antinuclear antibody (ANA) and antineutrophil cytoplasmic antibodies (ANCAs) were measured using an immunofluorescence assay. The level of rheumatoid factor (RF) was assessed by nephelometry (BN ProSpec system, Siemens AG, Henkestrasse, Erlangen, Germany). An enzyme-linked immunosorbent assay (ETI-Max 3000TM Microtiter Analyzer system; DiaSorin, Via Crescentino, Saluggia VC, Italy) was used to test for anti-cyclic citrullinated peptide (CCP), anti-Ro/SSA, anti-La/SSB, double-stranded DNA (dsDNA), Smith, Scl-70, ribonucleoprotein (RNP), histidyl-tRNA synthetase (Jo-1), proteinase 3 (PR3) and myeloperoxidase (MPO). The serological tests were considered positive if the circulating autoantibody levels were above the reference value, with the exception of ANA and RF, which were considered positive if the titers were ≥1:320 and 60 IU/mL, respectively (22).
Statistical analysis
Descriptive statistics are presented as the mean ± standard deviation or number (percentage). An unpaired Student’s t-test, Mann-Whitney rank sum test, chi-square test or Fisher’s exact test was used as appropriate for variable comparisons. To compare the survival between the IPF and CTD-UIP groups, Kaplan-Meier survival curves and the log-rank test were used to investigate the time from the first diagnosis to the last follow-up in the clinic or death (i.e., disease duration). The data obtained from the patients who had been lost to follow-up were considered censored observations. Unadjusted hazard ratios (HRs) were obtained for all study variables using a Cox proportional hazard regression analysis based on the mortality for the CTD-UIP and IPF/UIP patients. Adjusted HRs were used to identify the independent predictors of mortality for the CTD-UIP and IPF/UIP patients. A two-sided P value <0.05 and 95% confidence intervals (CIs) were used to report the statistical significance and precision of our results, respectively. The SPSS (Statistical Package for the Social Sciences) version 18 software package (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
One hundred fifty-five consecutive patients with UIP comprised the study cohort. Eighty-eight patients were diagnosed with IPF/UIP, and 67 patients were diagnosed with CTD-UIP.
Characteristics of CTD-UIP
The CTD subtypes in the established CTD-UIP cases included UCTD (n=22, 33%), rheumatoid arthritis (n=20, 30%), systemic lupus erythematosus (n=9, 13%), scleroderma (n=7, 10%), mixed CTD (n=4, 6%), Sjogren’s syndrome (n=4, 6%), and polymyositis/dermatomyositis (n=1, 2%). In the subset of the patients with UCTD, ANA was the most frequently positive autoantibody (n=17; 77%). Seventeen patients had ANA titers ≥1,280, and five patients had a titer of <160. Nine UCTD patients (41%) were positive for one antibody, seven patients (32%) were positive for two antibodies, and six patients (27%) had three or more positive serological tests. Comparisons of the demographic and clinical characteristics between the CTD and UCTD patients are shown in Table 1. A remarkable similarity with regards to age, gender, smoking status and disease duration was observed between the two groups. Ischemic heart disease was observed at a higher frequency in the UCTD group compared with the other CTDs (23% vs. 4%, P=0.042). However, there were no significant differences in the comorbidities or the treatment received between the groups. There were no differences in the PFT, 6MWT or ABG values between the UCTD patients and the patients with other CTDs (Table 2). None of the UCTD patients developed definite CTD during their follow-up at our clinic (median follow-up of 29 months).
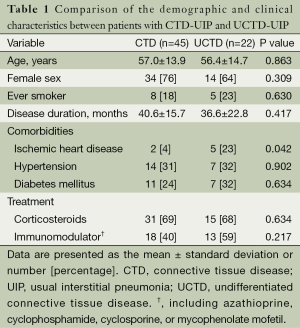
Full table
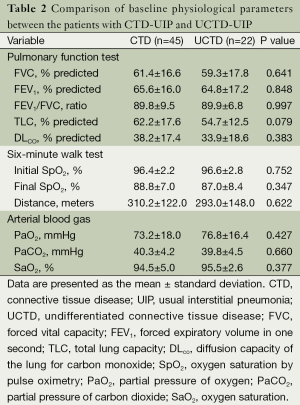
Full table
Comparison of demographic and clinical characteristics between IPF/UIP and CTD-UIP
The CTD-UIP patients were more likely to be young, female, and nonsmokers compared with the IPF/UIP group (Table 3). The disease duration was significantly longer for the CTD-UIP patients compared with the IPF/UIP patients (P=0.013). There were no significant differences in the comorbidities between the groups. A greater number of patients with CTD-UIP received corticosteroids and/or immunomodulators (e.g., azathioprine, cyclophosphamide, cyclosporine, or mycophenolate mofetil) compared with the IPF/UIP patients (P<0.0001). No differences in PFT indices were identified between the two groups (Table 4). Moreover, there was no significant difference in walking distance between the groups. However, the IPF/UIP patients had a significantly lower initial and final SpO2 during the 6MWT compared with the CTD-UIP patients (P<0.0001 and P=0.047, respectively). Furthermore, the IPF/UIP patients had a significantly lower PaO2 value (68.8±11.4 vs. 74.4±17.4 mmHg, P=0.017) compared with the CTD-UIP patients.
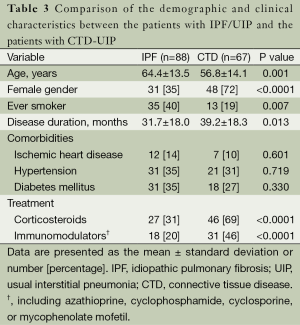
Full table
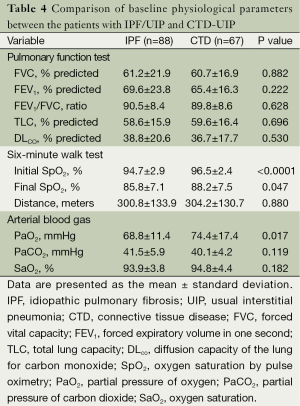
Full table
Comparison of the survival rate between IPF/UIP and CTD-UIP
During the study, there were 35 deaths [23 patients (26%) in the IPF/UIP group and 12 patients (18%) in the CTD-UIP group, P=0.225]. Of the patients who died in the CTD-UIP group, the CTD subtypes included UCTD (n=6), rheumatoid arthritis and mixed CTD (n=2 each), and systemic lupus erythematosus and scleroderma (n=1 each). There was no significant difference in survival between the patients with IPF/UIP or CTD-UIP (HR, 1.74; 95% CI, 0.86-3.50; P=0.113) (Figure 1). After removal of the UCTD subtype from the CTD-UIP cohort, a univariate Cox proportional hazards model analysis revealed that IPF/UIP was associated with a 2.47-fold increased risk of mortality compared with the CTD-UIP group (HR, 2.47; 95% CI, 1.01-6.09; P=0.049).
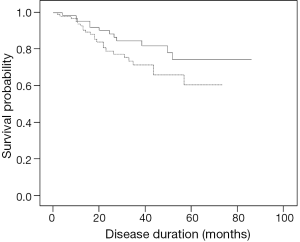
Multiple variables were associated with an increased risk of mortality in the CTD-UIP patients, including increasing age (HR, 1.09; 95% CI, 1.03-1.15; P=0.003) and the presence of ischemic heart disease (HR, 5.76; 95% CI, 1.72-19.20; P=0.004). In addition, a diagnosis of UCTD-UIP was associated with an increased risk of death with a trend towards significance (HR, 3.05; 95% CI, 0.96-9.63; P=0.057). In contrast, the variables associated with an improved survival for the UCTD-UIP group were female sex (HR, 0.32; 95% CI, 0.10-0.98; P=0.047), higher total lung capacity (for each 1% increase, HR, 0.95; 95% CI, 0.91-0.99; P=0.033), higher DLco (for each 1% increase, HR, 0.93; 95% CI, 0.87-0.98; P=0.014), higher initial SpO2 (for each 1% increase, HR, 0.79; 95% CI, 0.63-0.99; P=0.044), less oxygen desaturation during exercise (for each 1% increase, HR, 0.93; 95% CI, 0.87-0.99; P=0.032), higher walking distance during the 6MWT (for each 1 meter increase, HR, 0.99; 95% CI, 0.98-0.99; P=0.003), and higher SaO2 (for each 1% increase, HR, 0.90; 95% CI, 0.81-0.99; P=0.045).
In the IPF/UIP patients, a higher PaCO2 value was the only variable associated with an increased risk of death (for each 1 mmHg increase, HR, 1.11; 95% CI, 1.04-1.19; P=0.002). Conversely, the following variables were associated with improved survival: higher forced vital capacity (for each 1% increase, HR, 0.96; 95% CI, 0.93-0.98; P<0.0001), higher forced expiratory volume in one second (for each 1% increase, HR, 0.96; 95% CI, 0.94-0.98; P<0.0001), higher total lung capacity (for each 1% increase, HR, 0.95; 95% CI, 0.92-0.98; P<0.0001), higher initial SpO2 (for each 1% increase, HR, 0.78; 95% CI, 0.70-0.89; P<0.0001), less oxygen desaturation during exercise (for each 1% increase, HR, 0.88; 95% CI, 0.83-0.94; P<0.0001), higher walking distance during the 6MWT (for each 1 meter increase, HR, 0.99; 95% CI, 0.95-0.99; P=0.002), higher PaO2 (for each 1 mmHg increase, HR, 0.96; 95% CI, 0.92-0.99; P=0.034), and higher SaO2 (for each 1% increase, HR, 0.82; 95% CI, 0.75-0.90; P<0.0001).
A multivariate analysis using a Cox regression model revealed that age (HR, 1.11; 95% CI, 1.00-1.22; P=0.045) and walking distance (HR, 0.99; 95% CI, 0.98-1.00; P=0.044) were independent risk factors for survival in the patients with CTD-UIP. In the patients with IPF/UIP, a multivariate analysis revealed that total lung capacity (HR, 0.93; 95% CI, 0.88-0.99; P=0.017), final SpO2 during the 6MWT (HR, 0.88; 95% CI, 0.79-0.97; P=0.010), and SaO2 (HR, 0.64; 95% CI, 0.41-0.99; P=0.045) were independent predictors of survival.
Discussion
In this study, we found no apparent difference in survival between the CTD-UIP and IPF/UIP patients.
CTDs are a heterogeneous group of immunologically mediated inflammatory disorders with an unknown cause. They are characterized by multiple organ involvement with a variable clinical presentation, disease severity and outcome. Lung involvement is a common problem in CTDs, which leads to a significant increase in morbidity and mortality (7,23,24). Although nonspecific interstitial pneumonia (NSIP) is the most commonly reported pattern in CTD-associated ILD (CTD-ILD), other lung injuries, including UIP, are well documented with variable frequencies depending on the underlying form of CTD (25-28).
Previous studies (2-6) have reported conflicting results regarding the prognostic implications of CTD-associated lung fibrosis; however, this discrepancy may be explained because considerably different numbers of CTD subtypes were used in the studies. For example, 33% of our established CTD-UIP cases were a UCTD subtype, whereas the number of UCTD cases in the studies that reported a better survival for CTD-associated lung fibrosis was low (1%) (2-4). Importantly, 50% of the total deaths in our CTD-UIP cohort occurred within the UCTD subgroup. These data account for the absence of a significant difference in survival between the CTD-UIP and IPF/UIP patients. After removal of the UCTD subgroup from the CTD group, a univariate Cox proportional hazards model analysis revealed that IPF/UIP was associated with a 2.47-fold increased risk of mortality compared with CTD-UIP (HR, 2.47; 95% CI, 1.01-6.09; P=0.049). Thus, our data support previous studies that reported an improved survival for CTD-UIP patients (2-4).
A thorough search for systemic symptoms associated with autoimmune diseases and extensive serologic testing for patients who present with ILD is a routine practice in our center. Furthermore, we implemented a multidisciplinary approach that includes a pulmonologist, rheumatologist, radiologist and pathologist for all ILD cases before a final diagnosis was rendered. This approach potentially accounts for the substantial number of UCTD cases in our cohorts. Similarly, Castelino et al. (29) reported that the participation of rheumatologists in a multidisciplinary ILD clinic, which included a pulmonologist, radiologist and pathologist, changed the diagnosis of 54% of patients; moreover, therapies were changed in 80% of patients with CTD-ILD and 27% of patients with IPF. Thus, these data underscore the importance of a multidisciplinary approach to improve the accuracy of clinical diagnoses.
It is not uncommon for the lungs to be the primary site of initial clinical symptoms in CTDs, antedating the systemic manifestations by months or even years. Up to 25% of cases of chronic ILD have clinical and laboratory features suggestive of an autoimmune background, but they do not fulfill the classification criteria for well-defined CTD (30). More than three decades ago, LeRoy et al. (31) proposed the term UCTD to describe patients with symptoms and signs suggestive of autoimmune disease that do not fulfill the well-established criteria for CTDs. Consequently, the concept of UCTD became a distinct clinical entity characterized by symptoms and signs of autoimmune disease suggestive of CTD, a positive autoimmune serology and a disease duration of more than 1 year (16,17). Importantly, UCTD as described in the rheumatology literature has a mild clinical course, favorable prognosis and low prevalence of pulmonary fibrosis (1%) (17,31-33).
In contrast, in the pulmonology literature, UCTD appears to have a different clinical course. For example, Corte et al. (34) reported no survival advantage in patients with UCTD-ILD compared with patients without UCTD. In another study, Vij et al. (35) reported no significant difference in survival between autoimmune-featured ILD (of which 62% exhibited a typical UIP pattern on HRCT images) and IPF patients. Recently, Strand et al. (36) reported no significant difference in survival between UCTD-UIP and IPF patients. In the present study, we identified a trend towards significance for the diagnosis of UCTD-UIP associated with a 3.05-fold increased risk of death compared with the other CTD-UIP types (HR, 3.05; 95% CI, 0.96-9.63; P=0.057). Collectively, these data suggest that UCTD in the context of a UIP pattern is not associated with a favorable prognosis. However, a recent study by Kim et al. (37) reported a better prognosis for the UCTD-UIP (n=44) group compared with the IPF group (P=0.042). The reason for this discrepancy is unknown. One potential explanation is that our UCTD-UIP patients had a more severe physiologic impairment at diagnosis compared with the cohort studied by Kim et al. (37). The baseline physiological parameters of our patients included a percent predicted for FVC of 59.3±17.8, a percent predicted for DLCO of 33.9±18.6, and a distance measured by the 6MWT of 293.0±148.0 meters. In the patients studied by Kim et al. (37), the physiological parameters at baseline were of moderate severity (a percent predicted for FVC of 71.9±15.1, a percent predicted for DLCO of 55.3±16.0, and a distance measured by the 6MWT of 437.5±94.7 meters). Ethnic differences could explain the observed discrepancy. The patients in the Kim et al. (37) study were Asian, whereas our study consisted of Arab patients. Further studies with a larger number of UCTD-UIP patients from different populations with similar physiologic impairments are necessary.
The present study had several limitations. The retrospective review of a database from a single tertiary center may have introduced both selection and recall biases. In addition, the number of CTD patients in each subgroup was relatively small, and our study did not have sufficient power to perform a survival analysis or determine independent predictors of mortality for each CTD subtype. The amalgamation of patients with UIP caused by including various types of CTD in one group is potentially an important problem, as evidenced by the influence of the UCTD subgroup in our study. Therefore, further studies are needed to explore each CTD subtype and to improve our understanding of the natural history of specific disease patterns.
In conclusion, we demonstrated that the outcome of CTD-UIP patients was similar to IPF/UIP patients. However, the UCTD subgroup exerts an important negative impact on survival in CTD-UIP patients. Further studies are needed to validate our findings.
Acknowledgements
The work was conducted at King Khalid University Hospital, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
Disclosure: The author declares no conflict of interest.
References
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [PubMed]
- Park JH, Kim DS, Park IN, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007;175:705-11. [PubMed]
- Song JW, Do KH, Kim MY, et al. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest 2009;136:23-30. [PubMed]
- Navaratnam V, Ali N, Smith CJ, et al. Does the presence of connective tissue disease modify survival in patients with pulmonary fibrosis? Respir Med 2011;105:1925-30. [PubMed]
- Hubbard R, Venn A. The impact of coexisting connective tissue disease on survival in patients with fibrosing alveolitis. Rheumatology (Oxford) 2002;41:676-9. [PubMed]
- Kocheril SV, Appleton BE, Somers EC, et al. Comparison of disease progression and mortality of connective tissue disease-related interstitial lung disease and idiopathic interstitial pneumonia. Arthritis Rheum 2005;53:549-57. [PubMed]
- Solomon JJ, Ryu JH, Tazelaar HD, et al. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Respir Med 2013;107:1247-52. [PubMed]
- Kinder BW, Collard HR, Koth L, et al. Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med 2007;176:691-7. [PubMed]
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-24. [PubMed]
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271-7. [PubMed]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581-90. [PubMed]
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344-7. [PubMed]
- Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum 1993;36:340-7. [PubMed]
- Smolen JS, Steiner G. Mixed connective tissue disease: to be or not to be? Arthritis Rheum 1998;41:768-77. [PubMed]
- Doria A, Mosca M, Gambari PF, et al. Defining unclassifiable connective tissue diseases: incomplete, undifferentiated, or both? J Rheumatol 2005;32:213-5. [PubMed]
- Mosca M, Neri R, Bombardieri S. Undifferentiated connective tissue diseases (UCTD): a review of the literature and a proposal for preliminary classification criteria. Clin Exp Rheumatol 1999;17:615-20. [PubMed]
- Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720-35. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511-22. [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [PubMed]
- Fischer A, West SG, Swigris JJ, et al. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest 2010;138:251-6. [PubMed]
- Dankó K, Ponyi A, Constantin T, et al. Long-term survival of patients with idiopathic inflammatory myopathies according to clinical features: a longitudinal study of 162 cases. Medicine (Baltimore) 2004;83:35-42. [PubMed]
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis 2007;66:940-4. [PubMed]
- Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest 2005;127:2019-27. [PubMed]
- Bouros D, Wells AU, Nicholson AG, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 2002;165:1581-6. [PubMed]
- Douglas WW, Tazelaar HD, Hartman TE, et al. Polymyositis-dermatomyositis-associated interstitial lung disease. Am J Respir Crit Care Med 2001;164:1182-5. [PubMed]
- Parambil JG, Myers JL, Lindell RM, et al. Interstitial lung disease in primary Sjögren syndrome. Chest 2006;130:1489-95. [PubMed]
- Castelino FV, Goldberg H, Dellaripa PF. The impact of rheumatological evaluation in the management of patients with interstitial lung disease. Rheumatology (Oxford) 2011;50:489-93. [PubMed]
- Gutsche M, Rosen GD, Swigris JJ. Connective Tissue Disease-associated Interstitial Lung Disease: A review. Curr Respir Care Rep 2012;1:224-32. [PubMed]
- LeRoy EC, Maricq HR, Kahaleh MB. Undifferentiated connective tissue syndromes. Arthritis Rheum 1980;23:341-3. [PubMed]
- Mosca M, Tani C, Talarico R, et al. Undifferentiated connective tissue diseases (UCTD): simplified systemic autoimmune diseases. Autoimmun Rev 2011;10:256-8. [PubMed]
- Vaz CC, Couto M, Medeiros D, et al. Undifferentiated connective tissue disease: a seven-center cross-sectional study of 184 patients. Clin Rheumatol 2009;28:915-21. [PubMed]
- Corte TJ, Copley SJ, Desai SR, et al. Significance of connective tissue disease features in idiopathic interstitial pneumonia. Eur Respir J 2012;39:661-8. [PubMed]
- Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct entity. Chest 2011;140:1292-9. [PubMed]
- Strand MJ, Sprunger D, Cosgrove GP, et al. Pulmonary function and survival in idiopathic vs secondary usual interstitial pneumonia. Chest 2014;146:775-85. [PubMed]
- Kim HC, Ji W, Kim MY, et al. Interstitial Pneumonia Related to Undifferentiated Connective Tissue Disease: Pathologic Pattern and Prognosis. Chest 2015;147:165-72. [PubMed]


