Anaplastic lymphoma kinase-positive lung adenocarcinoma patient with development of sick sinus syndrome while on targeted treatment with crizotinib
Case report
A 46-year-old non-smoking woman presented with 3 months of cough and 2 months of intermittent hemoptysis. Her chest X-ray was right pleural effusion. She had weight loss but no chest pain, dyspnea and night sweats. On presentation to the department she had cough and hemoptysis with a rate of 8-10 mouth per day. Her physical examination showed right lower lobe low breath sounds without palpable lymphadenopathy. Carcinoembryonic antigen was within normal limits. Electrocardiogram (ECG) showed sinus bradycardia, heart rates (HR) was 58 beats per minute. CT scans of the chest demonstrated right hilar mass, right middle and lower lobe atelectasis (Figure 1A). Bronchoscope on day 3 demonstrated much fresh blood in right main bronchus, intermediate bronchial stenosis and mucosal hypertrophy, vision is not clear without biopsy (Figure 1B). Cranial MRI on day 4 showed two size of 1.1 cm ring shadow on the right side of the centrum semiovale, the edge is high T1, high T2 signal, a low T1 and high T2 signal in central, no edema around the lesions and boundary less clear (Figure 1C). Skeletal emission computed tomography (ECT) demonstrated the multiple bone metastasis. Transesophageal endoscopic ultrasonography guided mediastinal lymph node biopsy on day 5 showed low differentiation adenocarcinoma (Figure 1D).
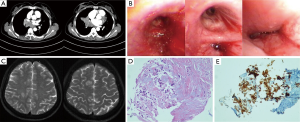
She was initially treated with pemetrexed and carboplatin/cisplatin for 4 cycles with stable disease. She did not have epidermal growth factor receptor (EGFR) gene testing and select gefitinib for maintenance treatment nearly 10 months. Chest CT demonstrated a little better in 2 months later, but hemoptysis and dyspnea became heavier in three months later. Pulmonary lesions aggravated three months after gefitinib therapy. The disease was progression with recurrent hemoptysis, after multidisciplinary team discussion a decision was made to carry out bronchial arterial embolism to reduce serious hemoptysis, but the main bronchial arterial was too narrow to super selective catheterization, then we gave up the intervention treatment.
The immunohistochemistry (IHC) showed anaplastic lymphoma kinase (ALK) overexpression for this patient (Figure 1E), so crizotinib was instead of gefitinib for continuing therapy. Before treatment, her ECG was normal (Figure 2A). After 40 days of therapy, her ECG was also normal (Figure 2B). The HR and blood pressure significantly decreased in the 41 days, the HR was 33 beats per minute, so we had to use vasoactive drugs to maintain blood pressure and HR. She carried out cranial irradiation after the emergence of dizziness, nausea and vomiting. After 14 times’ accumulated radiation, the symptoms of dizziness, nausea and vomiting were remission. Cranial MRI showed abnormal signals on the right side of the centrum semiovale 3 months after targeted therapy, the lesions are slightly increased.
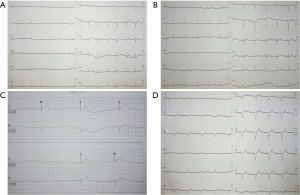
After 3 months of treatment, the manifestations of chest CT showed atelectasis and right lung infection was improving, pericardial effusion and right pleural effusion was increasing slightly. Holter showed sinus bradycardia, the longest R-R interval was 12.12 s, the slowest HR was 24 beats per minute (Figure 2C). She was diagnosed of sick sinus syndrome (SSS) and implanted pacemaker in April 22, 2014. She has ECG examination every 2-3 months after pacemaker implantation, which it shows pacing rhythm (Figure 2D). When the HR was lower than 60 beats per minute, the pacemaker will work. The patient remained alive, no loss of consciousness and oral crizotinib with regular follow-up when last seen in July 30, 2014.
Discussion
The mainstay of first-line treatment in patients with metastatic non-small cell lung cancer (NSCLC) whose tumors do not harbor an EGFR-activating mutation or an ALK translocation is doublet che¬motherapy. Pemetrexed-containing first-line regimens are well tolerated by most patients and are associated with a much lower incidence of neuropathy and cytopenias than many other regimens. She was initially treated with pemetrexed and platinum for four cycles with stable disease. Some studies showed switch maintenance with gefitinib for patients with advanced NSCLC not progressing after first-line chemotherapy would improve progression-free survival (PFS), but no statistically significant improvement in overall survival (OS) (1). The PFS of this patient was nearly three months, and it was shorter than previous reports. Some EGFR gene mutation patients involved in the previous studies may explain this difference.
Although the fluorescence in situ hybridization (FISH) was recommended the standard method for ALK gene testing, it was expensive, time consuming and difficult to interpret. Although standardized ALK-IHC protocols were missing, ALK protein expression would provide more clues for therapeutic strategies for NSCLC patients. The incidence of ALK rearrangement is only 3-5% in unselected NSCLC patients, several studies have reported that ALK-positive patients are younger and have never smoked (2,3). This patient was a young woman and never smoking, the pulmonary lesions were reduced after crizotinib treatment, but the central nervous system (CNS) was progressive, she underwent cranial irradiation. Some reports describe patients who continued to respond systemically in crizotinib treatment, but who progressed in the CNS (4,5). One possible explanation comes from a pharmacokinetic analysis in a single patient showing poor cerebrospinal fluid penetration (6). One study reported continuing treatment with crizotinib after progression disease (PD) would provide survival benefit to advanced ALK-positive NSCLC patients (7).
Crizotinib is associated with two main cardiac effects, QT interval prolongation and bradycardia. HR decreasing is a pharmacodynamic effect of crizotinib, averaging 2.5 fewer beats per minute with every 100 ng/mL increasing in the serum concentration of crizotinib (8). In the profile 1005 trial, a HR decreasing by a mean of 15.9 beats per minute was measured on day 22 of crizotinib treatment (9). In this report, the HR decreased significantly on day 41 after crizotinib treatment, the ECG monitoring revealed the HR was 33 beats per minute. After 3 months of treatment, her Holter showed bradycardia and RR prolongation, the lowest HR was 24 beats per minute and the longest time of RR was 12.12 s, then pacemaker was implanted to prevent sudden death. The exact mechanism of this phenomenon is not known. Some hypothesize that it might be related to c-Met inhibition (10). Physicians should be aware of these severe adverse effects when treating ALK positive NSCLC patients with crizotinib.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Gaafar RM, Surmont VF, Scagliotti GV, et al. A double-blind, randomised, placebo-controlled phase III intergroup study of gefitinib in patients with advanced NSCLC, non-progressing after first line platinum-based chemotherapy (EORTC 08021/ILCP 01/03). Eur J Cancer 2011;47:2331-40. [PubMed]
- Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009;15:5216-23. [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [PubMed]
- Takeda M, Okamoto I, Nakagawa K. Clinical impact of continued crizotinib administration after isolated central nervous system progression in patients with lung cancer positive for ALK rearrangement. J Thorac Oncol 2013;8:654-7. [PubMed]
- Chun SG, Choe KS, Iyengar P, et al. Isolated central nervous system progression on Crizotinib: an Achilles heel of non-small cell lung cancer with EML4-ALK translocation? Cancer Biol Ther 2012;13:1376-83. [PubMed]
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. [PubMed]
- Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 2014;25:415-22. [PubMed]
- Nickens D, Tan W, Wilner K, et al. Abstract 1673: A pharmacokinetics/pharmacodynamics evaluation of the concentration-QTc relationship of PF-02341066 (PF-1066), an ALK and c-MET/HGFR dual inhibitor administered orally to patients with advanced cancer. Cancer Res 2010;70:1673.
- Pfizer Canada. Xalkori. Crizotinib Capsules: Anaplastic Lymphoma Kinase (Alk) Tyrosine Kinase Inhibitor [product monograph]. Kirkland, QC: Pfizer Canada; 2012.
- Ou SH, Tong WP, Azada M, et al. Heart rate decrease during crizotinib treatment and potential correlation to clinical response. Cancer 2013;119:1969-75. [PubMed]


