
Challenges of PD-L1 testing in non-small cell lung cancer and beyond
Treatment options and clinical outcomes for non-small cell lung cancer (NSCLC) have recently been transformed with the introduction of immune checkpoint blockade (ICB), including antibodies targeting programmed cell death-1 (PD-1) and programmed death-ligand 1 (PD-L1). In advanced NSCLC patients with no epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) genomic tumour aberrations, anti-PD-1/PD-L1 given either as a monotherapy or combined with chemotherapy had superior efficacy over standard chemotherapy (1). Accordingly, nivolumab (OPDIVO, Bristol-Myers Squibb) (2); pembrolizumab (KEYTRUDA, Merck Sharp & Dohme) (3); atezolizumab (TECENTRIQ, Roche/Genentech) (4); and durvalumab (IMFINZI, MedImmune/AstraZeneca) (5) have achieved regulatory approval for advanced NSCLC (Table 1). These agents are expensive and have side effects that may lead to life-threatening toxicity. To maximise clinical benefits and spare unnecessary cost and toxicity, the challenge remains to stratify patients so that the most appropriate treatment options are selected. To achieve this, along with regulatory approval, immunohistochemistry (IHC) for PD-L1 expression was also approved as either a companion or complementary diagnostic test (Table 1).
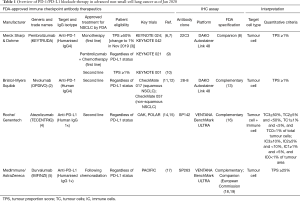
Full table
Although PD-L1 testing is now established as a routine clinical practice, challenges remain. Clinical trial data showed not all patients with high PD-L1 expression responded to PD-1/PD-L1 blockade, and responses were observed in PD-L1 low, or even PD-L1 negative patients (11,14,17,20). By contrast, biomarkers such as EGFR and ALK typically guide clinical management with clear binary results as either positive or negative. PD-L1 testing differs as the staining is often heterogeneous. PD-L1 is expressed transiently on various cell types in the tumour, and rarely only on cancer cells. More importantly, its expression can alter when tumour cells encounter immune effector cells in the tumour microenvironment and may be affected by previous treatment such as chemotherapy or targeted drug therapy. Another critical factor is the variable PD-L1 staining cut-off used in different clinical trials (6,7,9,10,21); this can affect the response rate. For example, in the KEYNOTE-024 trial (6), in which pembrolizumab was shown to be superior to platinum-based chemotherapy, the PD-L1 tumour proportion score (TPS) was ≥50%. However, in the CheckMate 026 trial, in which the TPS was ≥1% (21), there was no difference in the response rate. Furthermore, how PD-L1 staining is quantified can be influenced by various factors including site and method of biopsy, which antibody is used, and the staining methodology, including which platform is used in the IHC assay and which cell type/s are included in the analysis (Figure 1). This review will primarily discuss these major challenges of PD-L1 testing in clinical practice and update the results regarding biomarker analysis in NSCLC.
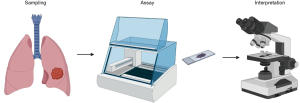
Sampling
Spatial heterogeneity
The staining pattern indicating PD-L1 expression can show both intra-tumoral and inter-tumoral heterogeneity. Therefore, PD-L1 expression status can be significantly impacted by the sampling method: surgical resection or biopsy. Several studies reported discordant PD-L1 status between resected and biopsied tissue. A comparison of PD-L1 expression in 160 NSCLC patients revealed a significant discordance between pre-operative biopsy and their corresponding resected specimens (22). In another study, 14% of cases demonstrated discordance of PD-L1 expression between biopsy cores and surgically resected specimens (23). These results highlight that a single biopsy may be insufficient to accurately capture PD-L1 status and emphasise the importance of using a surgically resected specimen when possible. Biopsy specimens obtained through transbronchial needle aspiration or transbronchial biopsy are sometimes the only available samples for advanced NSCLC; this can be a potential issue for appropriately recommending a therapeutic strategy that includes PD-1/PD-L1 blockade.
Differences in PD-L1 expression between primary and metastatic sites might also be a potential issue. Mansfield et al. (24) evaluated PD-L1 expression in paired lung cancer lesions from 32 patients with multifocal lung cancer, and showed strong consistency in tumour cell PD-L1 expression among different pulmonary metastases (24). Kim et al. demonstrated the concordance rate of PD-L1 expression between primary and metastatic lesions from 146 lung cancer to be 80.1% and 90.7% using cut-offs of 1% and 50%, respectively (25). These results suggest that differences between primary and metastatic samples, or between different metastases in the same organ are less significant when comparing biopsy and surgical resection.
Temporal heterogeneity
PD-L1 expression changes during a patient’s clinical course; this occurs spontaneously and is also influenced by treatment including surgical resection, systemic chemotherapy or targeted therapy (26). Sheng et al. (11) reported PD-L1 expression on tumour cells changed from 75% to 37.5% after paclitaxel-based, pemetrexed-based or tyrosine kinase inhibitor-based neoadjuvant chemotherapy in NSCLC patients. In contrast, a separate study found 30.2% of NSCLC patients changed PD-L1 tumour expression from negative to positive after platinum-based neoadjuvant chemotherapy (12). Thus, it is critical to evaluate PD-L1 expression in serial samples throughout the treatment. Furthermore, for heavily treated NSCLC patients, evaluating the PD-L1 expression in the most recent tumour specimen is necessary before making a decision to use PD-1/PD-L1 blockade on these patients.
Archived or newly collected tissue
Currently, PD-L1 testing is performed mainly using archived formalin-fixed, paraffin-embedded (FFPE) specimen, potentially raising concerns as to whether antigenicity is preserved in these samples. However, a recent analysis of the KEYNOTE-010 trial (27) compared patient outcomes based on PD-L1 expression in 455 archival and 578 newly collected tumour specimens, and demonstrated that the hazard ratios for survival were similar in the two groups (27). This result indicates that both archival and newly collected specimens are suitable for PD-L1 IHC testing.
Assay
Four commercial assays that utilise four different antibody clones (SP142, SP263, 22C3, and 28-8) on two different IHC platforms (Dako and Ventana) are currently available for measuring PD-L1 expression in FFPE specimen (Table 1). For example, to use pembrolizumab as first-line treatment in patients with metastatic NSCLC, PD-L1 expression on tumour cells is required to be TPS ≥1% (3), as determined by the Dako 22C3 pharmDx companion test (8). FDA has approved nivolumab with Dako 28-8 pharmDx as its complementary PD-L1 diagnostic assay (13). The European Commission approved Ventana SP263 as a companion diagnostic test for durvalumab (18,19) in Stage III NSCLC with TPS ≥1%. Ventana SP142 was approved by the FDA as a complementary assay for Atezolizumab (16).
It is clinically impractical to perform multiple tests to detect PD-L1 expression in addition to the required biomarker testing, such as EGFR and ALK, when only limited resources are available, especially when only a small biopsy is available. To standardise the various PD-L1 assays, the International Association for the Study of Lung Cancer has launched the Blueprint project. As reported, the Dako 22C3, Dako 28-8 and Ventana SP263 assays showed a high concordance across a broad range of thresholds; however, the Ventana SP142 assay recorded a lower expression of PD-L1 on tumour cells (28). This suggested that some PD-L1 assays (22C3, 28-8, and SP263) could be potentially used interchangeably (29). However, there is as yet no ‘gold standard’ assay that most accurately measures PD-L1 expression, and best predicts the clinical response to PD-1/PD-L1 blockade (30).
In terms of automated staining platforms, a recent study evaluated PD-L1 expression in the three main commercially available autostainers: Ventana BenchMark ULTRA, Dako Autostainer Link 48, and Leica Biosystems Bond-III. The study observed 100% concordance with dichotomised TPS results using the Dako Autostainer Link 48 and Ventana BenchMark ULTRA platforms (31). This suggests Dako and Ventana platforms can be used interchangeably.
Interpretation
Apart from different antibody clones and staining platforms, the four assays have unique staining patterns, scoring systems, and thresholds to quantitate PD-L1 expression. This makes the interpretation of PD-L1 IHC assays a clinical challenge. There is a difference in which cell types were evaluated as target cells for PD-L1 expression (Table 1). For 22C3, 28-8 and SP263 assay, tumour cells are the target to define positive PD-L1 staining. However, in the Ventana SP142 assay, both tumour cells (TC) and tumour-infiltrating immune cells (IC) are scored. TC were scores as a percentage of total tumour cells (TC3≥50%, TC2≥5% and <50%, TC1≥1% and <5%, and TC0<1%), and IC were scored as a percentage of tumour area (IC3≥10%, IC2≥5% and <10%, IC1≥1% and <5%, and IC0<1%) (15).
There are also variable cut-offs used to define a tumour as PD-L1 positive (Table 1). In the Ventana SP263 assay, samples were considered positive if ≥25% of tumour cells showed membrane staining of PD-L1. While in the Dako 28-8 assay, samples were classified as positive when at least 1% of tumour cells had membrane staining (12). Furthermore, the threshold may be distinct depending on the line of treatment. For example, pembrolizumab with its companion Dako 22C3 assay was approved as monotherapy in the first-line setting for advanced NSCLC patients with ≥50% (10) [changed to 1% in Nov 2019 (3)] tumour cells expressing PD-L1. While in the second-line setting for advance NSCLC patients, the 22C3 assay cut-off is 1% to prescribe pembrolizumab (10). In summary, the choice of anti-PD-1/PD-L1 drug selects the specific companion/complementary assay, each of which has different assay cut-offs and interpretation.
Beyond FFPE specimen: cytological specimens, peripheral blood, or imaging
Cytology specimens
To date, cytology samples are excluded for PD-L1 assessment in clinical settings due to the lack of tissue architecture—histological samples remain the only patient material permitted to assess PD-L1 IHC for admission to clinical trials. However, cytology samples are often the only materials available in patients with metastatic disease who do not undergo biopsy or surgical resection. To explore this issue further, PD-L1 expression was investigated on paired cytology and histology samples. A recent study showed a high degree of consistency between 86 paired histological and cytological samples (32); this finding was confirmed in a separate study (33). In an NSCLC cohort which included cytology cell blocks, small biopsies and surgical specimens (more than 200 samples of each type) the Dako 22C3 assay was used to evaluate PD-L1 expression. This showed PD-L1 IHC worked well on cytology cell blocks when TPS ≥50% was set as the endpoint (34). These studies demonstrate the potential value of cytological specimens for PD-L1 testing in advanced NSCLC when histological samples are unavailable.
Peripheral blood
A recent study explored the possibility of using peripheral blood as a non-invasive method to assess PD-L1 status in advanced NSCLC patients (35). In a further study of 71 specimens with matched circulating tumour cells (CTCs), concordance between tumour tissue and CTCs was 93%. Among 74 tumour samples with matched circulating white blood cells (WBCs), 54% of the patients revealed ≥1% PD-L1-positive immune cells in tumour tissue and 53% of the patients showed ≥1% PD-L1 positive in WBCs. This data reflects a high correlation (80%) between tissue and blood samples, and suggests testing peripheral blood for PD-L1 expression is a non-invasive and real-time method to assess PD-L1 expression in advanced NSCLC patients.
Imaging
PET-CT has been used as a potential non-invasive method for PD-L1 expression in NSCLC patients (36-38). Niemeijer et al. (36) reported the results of whole-body PET imaging using 18F-BMS-986192 and 89Zr-Nivolumab before nivolumab treatment in advanced NSCLC, the first-in-human study of this kind. Heterogeneity of tracer uptake was observed both between patients and within different tumours of the same patient. Using NM-01, a single-domain antibody against PD-L1, radio-labelled at a specific site with 99mTc for SPECT imaging, Xing et al. (38) demonstrated tumour uptake to be readily detectable against background tissues. This finding was particularly observed two hours after infusion, when the target-to-background (TBR) ratio correlated with PD-L1 immunohistochemistry results. These results suggest that there is potential to use radioactive tracers to both non-invasively and longitudinally quantify the expression level of PD-L1 in the future; however, larger datasets are necessary to validate the approach.
Biomarkers beyond PD-L1
As mentioned above, PD-L1 IHC is not an ideal assay for deciding whether ICB should be recommended—discovery and validation of novel predictive biomarkers is required. Various surrogate biomarkers are currently under intense investigation, including tissue tumour mutation burden (TMB) (39), immune gene signatures (40) and the presence, localisation and activation status of tumour-infiltrating lymphocytes (41). In addition, T-cell receptor diversity (42) and gut microbiome (43) have garnered interest as emerging biomarkers for ICB efficacy but have to be validated in large clinical studies. Blood-based biomarkers, such as neutrophil-to-lymphocyte ratio (NRL) (44) and blood-TMB (45), are also showing promise.
Of the above alternatives, TMB is currently the most promising predictive biomarker. Tumour somatic mutations can potentially give rise to neoantigens, which results in increased immunogenicity. In support of this hypothesis, data from the KEYNOTE-010 and -042 studies showed associations between high TMB (≥175 mutations per exome) and improved clinical outcome with pembrolizumab monotherapy in NSCLC patients (46). However, analysis of the KEYNOTE-021, -189 and -407 trials showed TMB was not significantly associated with the efficacy of first-line pembrolizumab plus platinum-based chemotherapy or with chemotherapy alone in metastatic NSCLC (47). Further clinical studies are required to determine a conclusive outcome of this approach.
As with PD-L1 testing, implementing TMB assays in clinical practice also faces many challenges: sequencing platform selection, bioinformatic algorithm development, and threshold setting. For sequencing platform, whole-exome sequencing (WES) is considered to be the standard for TMB assessment (20), as it covers the coding regions of ~22,000 genes (~1% of the genome, ~30 Mb). However, it is challenging to apply WES in a clinical setting due to its cost, turnaround time, and the requirement for complex analysis (48). Targeted sequencing panels, such as FoundationOne CDx (324 genes, ~0.8 Mb) and MSK-IMPACT (468 genes, ~1.5 Mb), offer a reasonable estimate of TMB from the whole-exome (49). Apart from differences in sequencing broadness, sequencing depth also varies with ≥500× for targeted panels and ≥100× for WES. Another critical variation in TMB assessment across studies is bioinformatic analysis pipelines, which are often not publicly reported (50). Different analyses may differ in which type of mutation to include or discard, such as insertion and deletion alterations (indels), as well as synonymous and non-synonymous mutation. A further important variable is a threshold set for defining the TMB level. To date, only a TMB threshold of ≥10 mutations/Mb using the FoundationOne CDx platform is established for NSCLC patients (39).
Despite these important caveats, the first TMB assay approved by FDA incorporated WES data from the patient tumour as well as matched normal tissue (e.g., peripheral blood); this FDA approval occurred in Nov 2019 (51). Further efforts to ensure reproducible results and reporting will facilitate the smooth implementation of TMB testing for ICB therapy. Meanwhile, several studies have compared TMB and PD-L1 expression for their predictive capacity (39,49)—it has been consistently shown that although TMB is independent of PD-L1 expression, it has similar predictive capacity. Further development of a predictive model incorporating both PD-L1 IHC expression and TMB could potentially yield higher predictive power.
Conclusions
Spatial and temporal heterogeneity, as well as many technical differences in methods used to assess PD-L1 expression, are major obstacles for consistency in PD-L1 testing, and may account for the suboptimal correlation of PD-L1 expression and response rates to PD-1/PD-L1 blockade in NSCLC. There remains a great need to standardise sample quality control, PD-L1 testing reagents and IHC platforms to definitively assess the interchangeability of the various tests, and to reach consensus on guidelines that will guide practice internationally. In conclusion, it is currently clear that no single test is a reliable predictive biomarker for immunotherapy. Future studies investigating predictive algorithms built on tumour-host interaction data could better stratify patients for immunotherapy.
Acknowledgments
Figure 1 in this manuscript was created with BioRender.com.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Clare Y. Slaney, Jian Zhang and Peng Luo) for the series “Immunotherapy and Tumor Microenvironment” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-2019-itm-010). The series “Immunotherapy and Tumor Microenvironment” was commissioned by the editorial office without any funding or sponsorship. PJN reports grants from Roche Genetech, grants from BMS, grants from Allergan, grants from Compugen, grants from Juno-Celgene, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu T, Ding S, Dang J, et al. First-line immune checkpoint inhibitors for advanced non-small cell lung cancer with wild-type epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK): a systematic review and network meta-analysis. J Thorac Dis 2019;11:2899-912. [Crossref] [PubMed]
- FDA US. Modification of the Dosage Regimen for Nivolumab. In: FDA US, editor. 09/15/2016.
- FDA US. FDA expands pembrolizumab indication for first-line treatment of NSCLC (TPS ≥1%). In: FDA US, editor. 04/11/2019.
- FDA US. FDA approves atezolizumab with nab-paclitaxel and carboplatin for metastatic NSCLC without EGFR/ALK aberrations. In: FDA US, editor. 3/12/2019.
- FDA US. FDA approves durvalumab after chemoradiation for unresectable stage III NSCLC. In: FDA US, editor. 02/20/2018.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- US Food and Drug Administration. Dako PD-L1 IHC 22C3 pharmDx. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf15/P150013c.pdf
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- US Food and Drug Administration. Dako PD-L1 IHC 28-8 pharmDx. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf15/P150025c.pdf
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- US Food and Drug Administration. VENTANA PD-L1 (SP142) Assay. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160002c.pdf
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- US Food and Drug Administration. VENTANA PD-L1 (SP263) Assay. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160046C.pdf
- AstraZeneca. European Commission approves Imfinzi for locally-advanced, unresectable NSCLC. Available online: https://www.astrazeneca.com/media-centre/press-releases/2018/european-commission-approves-imfinzi-for-locally-advanced-unresectable-nsclc-24092018.html
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 2016;27:147-53. [Crossref] [PubMed]
- Kim H, Kwon HJ, Park SY, et al. PD-L1 immunohistochemical assays for assessment of therapeutic strategies involving immune checkpoint inhibitors in non-small cell lung cancer: a comparative study. Oncotarget 2017;8:98524-32. [Crossref] [PubMed]
- Mansfield AS, Murphy SJ, Peikert T, et al. Heterogeneity of Programmed Cell Death Ligand 1 Expression in Multifocal Lung Cancer. Clin Cancer Res 2016;22:2177-82. [Crossref] [PubMed]
- Kim S, Koh J, Kwon D, et al. Comparative analysis of PD-L1 expression between primary and metastatic pulmonary adenocarcinomas. Eur J Cancer 2017;75:141-9. [Crossref] [PubMed]
- Shin J, Chung JH, Kim SH, et al. Effect of Platinum-Based Chemotherapy on PD-L1 Expression on Tumor Cells in Non-small Cell Lung Cancer. Cancer Res Treat 2019;51:1086-97. [Crossref] [PubMed]
- Herbst RS, Baas P, Perez-Gracia JL, et al. Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: an updated analysis of KEYNOTE-010 trial. Ann Oncol 2019;30:281-9. [Crossref] [PubMed]
- Adam J, Le Stang N, Rouquette I, et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol 2018;29:953-8. [Crossref] [PubMed]
- Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051-8. [Crossref] [PubMed]
- Mayo Medical Laboratories. PD-L1 immunohistochemitry options. Available online: https://news.mayocliniclabs.com/n1/96e99366cea7b0de/uploads/2018/09/PD-L1-flyer-0918.pdf
- Ilie M, Khambata-Ford S, Copie-Bergman C, et al. Use of the 22C3 anti-PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PLoS One 2017;12:e0183023. [Crossref] [PubMed]
- Skov BG, Skov T. Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol 2017;25:453-9. [Crossref] [PubMed]
- Heymann JJ, Bulman WA, Swinarski D, et al. PD-L1 expression in non-small cell lung carcinoma: Comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol 2017;125:896-907. [Crossref] [PubMed]
- Wang H, Agulnik J, Kasymjanova G, et al. Cytology cell blocks are suitable for immunohistochemical testing for PD-L1 in lung cancer. Ann Oncol 2018;29:1417-22. [Crossref] [PubMed]
- Ilie M, Szafer-Glusman E, Hofman V, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol 2018;29:193-9. [Crossref] [PubMed]
- Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun 2018;9:4664. [Crossref] [PubMed]
- Takada K, Toyokawa G, Yoneshima Y, et al. (18)F-FDG uptake in PET/CT is a potential predictive biomarker of response to anti-PD-1 antibody therapy in non-small cell lung cancer. Sci Rep 2019;9:13362. [Crossref] [PubMed]
- Xing Y, Chand G, Liu C, et al. Early Phase I Study of a (99m)Tc-Labeled Anti-Programmed Death Ligand-1 (PD-L1) Single-Domain Antibody in SPECT/CT Assessment of PD-L1 Expression in Non-Small Cell Lung Cancer. J Nucl Med 2019;60:1213-20. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930-40. [Crossref] [PubMed]
- Mazzaschi G, Madeddu D, Falco A, et al. Low PD-1 Expression in Cytotoxic CD8(+) Tumor-Infiltrating Lymphocytes Confers an Immune-Privileged Tissue Microenvironment in NSCLC with a Prognostic and Predictive Value. Clin Cancer Res 2018;24:407-19. [Crossref] [PubMed]
- Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91-7. [Crossref] [PubMed]
- Postow MA, Manuel M, Wong P, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer 2015;3:23. [Crossref] [PubMed]
- Takeda T, Takeuchi M, Saitoh M, et al. Neutrophil-to-lymphocyte ratio after four weeks of nivolumab administration as a predictive marker in patients with pretreated non-small-cell lung cancer. Thorac Cancer 2018;9:1291-9. [Crossref] [PubMed]
- Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018;24:1441-8. [Crossref] [PubMed]
- Herbst RS, Lopes G, Kowalski DM, et al. Association between tissue TMB (tTMB) and clinical outcomes with pembrolizumab monotherapy (pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Ann Oncol 2019;30:v916-7. [Crossref]
- Paz-Ares L, Langer CJ, Novello S, et al. Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) for metastatic NSCLC: Tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Ann Oncol 2019;30:v917-8. [Crossref]
- Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31:1023-31. [Crossref] [PubMed]
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol 2018;36:633-41. [Crossref] [PubMed]
- Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [Crossref] [PubMed]
- FDA Clears NantHealth Tumor-Normal Whole-Exome Test. Available online: https://www.genomeweb.com/regulatory-news-fda-approvals/fda-clears-nanthealth-tumor-normal-whole-exome-test


