Clinical application of enhanced recovery after surgery (ERAS) in pectus excavatum patients following Nuss procedure
Introduction
Pectus excavatum (PE) is the most common congenital chest wall deformity and is characterized by depression of the anterior chest wall and sternum (1,2). The cause of PE is unclear, and the incidence is about 1/1,000–1/400 (3). It can be cosmetically problematic for patients and result in cardiopulmonary symptoms like exercise intolerance, fatigue, dyspnea, and chest pain (4,5). Surgery is the only effective way to cure the disease. Since the introduction of the minimally invasive repair of PE in 1998 by Nuss, it has become generally adopted by pediatric and thoracic surgeons as it entails less trauma, fewer postoperative complications, and better cosmetic outcomes (6).
Enhanced recovery after surgery (ERAS) protocols have been utilized in abdominal, urinary, and thoracic surgery in order to provide optimal care in the perioperative period, with the aim of shortening time to full recovery, and have been shown to be safe and effective (7-9). However, there are few reports on the clinical application of ERAS in Nuss surgery (10). In this study, the perioperative management of patients with pectus excavatum was conducted by ERAS strategy and traditional procedure to compare the effects on clinical outcomes. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1516).
Methods
After obtaining appropriate ethics approval from the Liaoning Cancer Hospital Ethics Committee, a retrospective study involving 168 patients with pectus excavatum collected from the Department of Thoracic Surgery of The Cancer Hospital of China Medical University between September 2016 and September 2019 was performed. These patients were divided into 2 groups by perioperative management: the traditional procedure group (T group) and the ERAS strategy group (E group). Basic demographics were obtained and included age, gender, body mass index (BMI), Haller index, operative time, postoperative drainage time, postoperative hospital time, and postoperative complications. Operative time was defined as the time between the beginning of the first port incision to wound suture completion; postoperative drainage time was defined as the time from the next day of the operation to the day of chest drainage tube removal; postoperative hospital time was defined as the time from the next day of the operation to last day at the hospital.
Inclusion criteria
An operation was indicated if 2 or more of the following criteria applied: chest computed tomography (CT) showed cardiac and pulmonary compression or both and a CT index of 3.25 or greater; cardiology evaluation demonstrated cardiac compression, displacement, mitral valve prolapse, murmurs or conduction abnormalities; pulmonary function study showed restrictive and/or obstructive lung disease; previous repair had failed; the thoracic deformity developed gradually; patients and their family members suffered psychologically and needed to correct the appearance (11). The main steps of the operation were performed by the same surgeon.
Exclusion criteria
The exclusion criteria were as follows: patients with comorbidities and requiring thoracotomy; patients with anterior chest wall severe asymmetry and depression; chest CT showing a CT index <3.0 and mild depression patients without related symptoms; complex patients with other thoracic deformities; patients with severe scoliosis; patients with Marfan syndrome and the skin or soft tissue infection near the incision (12,13). Patients were also excluded if the medical record was not complete.
Preoperative preparation
All patients were required to undergo routine preoperative laboratory tests, chest DR, chest CT and, cardiopulmonary function test. Once the cardiopulmonary function test outcome was abnormal, relevant tests were required to assess the surgical risk. The patients of ERAS group wore diapers into the operating room on the day of operation.
Perioperative management measures
Perioperative management measures for the traditional procedure group were as follows: patients were under tracheal intubation anesthesia, and had an intraoperative indwelling urinary catheter (IDUC) and indwelling drainage of the right pleural cavity with a 28-F chest tube. The measures for the ERAS strategy group were as follows: patients were under laryngeal mask airway (LMA) for non-endotracheal-intubated anesthesia, were wearing diapers instead of having an intraoperative indwelling of catheter, and had indwelling drainage of the right pleural cavity or subcutaneous with a 15-F drainage.
Postoperative results criteria
The treatment outcome grading proposed by Croitoru in 2005 was adopted for this study (14): excellent, if preoperative symptoms were resolved and chest appearance was normal; good, if preoperative symptoms were resolved and chest appearance was improved; fair, if preoperative symptoms were improved but the appearance was not completely normal; and failed, if symptoms were worse and chest appearance was not improved or if the deformity reoccurred.
Postoperative complications
Postoperative complications can be divided into early complications and late complications. The early complications included pneumothorax, pleural effusion, and poor incision healing. The late complications included plate displacement, rejection, and pectus excavatum recurrence (15). In this study, the collected postoperative complications were all those that needed to be treated clinically, including pneumothorax and pleural effusion requiring puncture or indwelling closed drainage.
Clinical outcomes
The primary outcome measures were postoperative drainage time and length of stay in days with the day of operation recorded as day 0. Postoperative complications measured by the Clavien-Dindo method were recorded as secondary outcomes.
Statistical analysis
Statistics of the collected data are mainly represented by the numbers and percentages, mean and standard deviations (SD), or the median and interquartile range (IQR). Continuous variables were depicted based on IQR and the median or SD and mean. Wilcoxon’s rank-sum test was used for ranked variables, and Two-sample Student’s t-test was used to compare the means of population between 2 different continuous variables. Chi-squared or Fisher’s exact test was also used to evaluate categorical variables that had statistically significant differences. All hypothesis tests were two-sided, with a P value of <0.05 considered a statistically significant difference.
Results
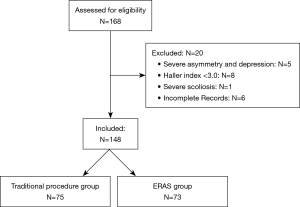
Between September 2016 and September 2019, there were 168 pectus excavatum patients. Of these, 5 were excluded due to severe asymmetry and depression, 8 were excluded due to having a Haller index <3.0, 1 was excluded due to severe scoliosis, and 6 were excluded due to incomplete medical records (Figure 1). This left a total of 148 patients, 75 of whom underwent the traditional procedure (T group), and 73 of whom underwent the ERAS strategy (E group).
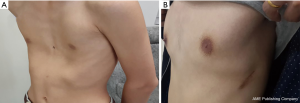
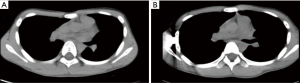
All operations involved in this study were completed successfully. None of patients in group E required conversion to an endotracheal tube because of continuous air leak. Postoperative follow-up was obtained after a median of 36.6 months (range, 2–46 months). In Group T, 73 patients’ chest wall deformities were treated successfully, and the satisfaction rate was 97%; in Group E, 72 patients’ chest wall deformities were treated successfully, and the satisfaction rate was 99% (Figures 2,3).
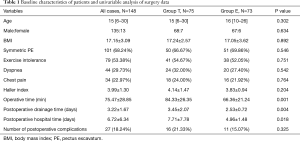
Demographics and the clinical laboratory characteristics are shown in Table 1. There were 135 male and 13 female patients. Patients ranged from 6 to 30 years (median, 15 years). No statistically significant correlations (P value >0.05) were observed in the 2 groups between age, sex, BMI, symmetric PE, exercise intolerance, dyspnea, chest pain, Haller index, or number of postoperative complications. In group E, the operative time (66.36±21.24 vs. 84.33±26.35 minutes), postoperative drainage time (2.53±0.72 vs. 3.45±2.07 days), and postoperative hospitalization time (4.96±1.48 vs. 7.71±7.78 days), were significantly better than those in group T (P<0.05).
Full table
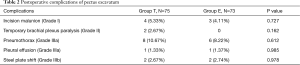
Postoperative complications of pectus excavatum are available in Table 2. There were 16 cases (21.33%) with minor postoperative complications in group T and 11 cases (15.07%) in Group E. There were no differences in overall complications as measured by Clavien-Dindo score. The early complications included pneumothorax, incision malunion, pleural effusion, and temporary brachial plexus paralysis. The pneumothorax patients were recovered after indwelling drainage, patients with incision malunion were recovered after regular incision dressing and adjustment of the antibiotics, patients with pleural effusion were recovered after the puncture, and patients with brachial plexus paralysis were recovered after treatment of anti-inflammatory symptoms and physical therapy. The long-term complications mainly included steel plate shift, and all patients recovered successfully after the second Nuss procedure.
Full table
Discussion
Based on evidence-based medicine, ERAS optimizes the clinical pathway of perioperative management through the cooperation of multiple departments including surgery, anesthesia, and nursing, so as to alleviate perioperative stress response, shorten hospital stay, and promote patients’ recovery (16). The implementation of an ERAS-related pathway can help improve the safety and satisfaction during the perioperative period, shorten postoperative hospital stay, and thus reduce medical costs (17,18). However, it remains controversial as to whether the current ERAS strategy can achieve good effects in children (19). Studies have shown that the selection of one or more appropriate ERAS measures can also benefit the postoperative recovery of children without forcing the use of a complete set of ERAS management plans (20). In this study, several ERAS strategies were applied to group E during the perioperative period, covering the preoperative, intraoperative, and postoperative periods, with the patients in group E having an orthopedic satisfaction rate of 99%, which is slightly higher than the 96% reported internationally (21).
The presence of an IDUC is routine following thoracic resections, but Roberts (22) believed that no IDUC following colectomy was associated with a reduction in intravenous fluid (IVF) administration and length of stay. In his study, patients were divided into cohorts based on the presence or absence of an IDUC upon leaving the operating theatre; fluid administration was lower in the group without routine IDUC, while length of stay was lower in the “no IDUC” group with no difference in morbidity and mortality. The age of PE patients is relatively low, and intraoperative IDUC could lead to obvious stimulation to the urethral mucosa, which is more likely to cause postoperative urethral pain or bleeding, and may also increase the burden of patients’ activities. In the study, patients in group E wore diapers into the operating room on the day of operation and none of them needed postoperative IDUC due to dysuria, which avoided the urethral stimulation altogether, as opposed to merely reducing it. Patients could take the functional exercise as soon as possible which accelerated the recovery and shortened the postoperative hospital time.
In this study, laryngeal mask anesthesia was used to replace endotracheal intubation anesthesia in the group E patients, and none of them required conversion to an endotracheal tube. In the past, single-chamber endotracheal intubation anesthesia was routinely used in Nuss surgery, and low ventilator tidal volume was performed during the operation (23). Liu et al. (24) reported that the general anesthesia of laryngeal mask replacing a single lumen tracheal tube did not increase the risk of anesthesia. Du et al. (25) believed that the use of LMA for non-endotracheal intubated anesthesia for selected patients with PE undergoing thoracoscopic Nuss procedure is clinically safe and technically feasible. In our study, patients in group E used laryngeal mask instead of endotracheal intubation. All these cases were completed successfully, which reduced the anesthesia time to some extent, significantly improved postoperative laryngeal pain and other discomfort, improved the satisfaction during perioperative period, shortened the hospital time, and reduced the hospital cost.
The routine use of postoperative pleural cavity drainage after the Nuss procedure is not widely accepted, and its limited use depends on experience. Pawlak et al. (26) reported their study of analyzing the influence of pleural drainage in the Nuss procedure treatment of 103 patients with pectus excavatum on the prevention of pneumothorax and the efficacy of using drainage after a corrective operation from November 2013 to May 2015. They found that patients without drainage manifested more complications in the early postoperative period, and pneumothorax requiring additional chest tube placement was statistically significant with other complications also being more frequent. They concluded that routine drainage of the pleural cavity during the Nuss procedure significantly reduces the incidence of postoperative pneumothorax and should be considered as a routine procedure. In our study, patients in group E had indwelling drainage of the right pleural cavity or subcutaneously with a 15-F drainage tube instead of in the right pleural cavity with a 28-F chest tube. The incidence of postoperative pneumothorax and pleural effusion in group E was decreased when compared to group T but without statistical significance. In general, drainage during the Nuss procedure is routine. We believe that tubule drainage can alleviate pain and reduce pleural exudation by limiting the pleural stimulation, thereby decreasing the days of drainage and the hospital time, and ultimately cutting hospital expenses.
Wharton et al. (10) protocol standardized perioperative exercise and pharmacologic regimens, pre- and post-operative education, and early return to activity, reported that implementation of ERAS for the Nuss procedure leads to a significant reduction in LOS, early pain scores, and urinary catheter usage, without an increase in post-operative ED visits and hospital readmissions, and believed an ERAS protocol should be utilized in this patient population. Their view is somewhat similar to that of this study, but there are differences between the two studies. Firstly, there are a considerable proportion of patients needed postoperative IDUC in their study, and in our study, patients in group E did not appear as postoperative IDUC due to dysuria. Secondly, none of their interventions involved anesthesia, but in our study, patients in group E used laryngeal mask instead of endotracheal intubation, which reduce the anesthesia time, decrease the stimulation of airway and discomfort.
In our study, the measures of no IDUC, laryngeal mask anesthesia, and indwelling tubule drainage can benefit the postoperative recovery of pectus excavatum patients. The possible mechanism of these measures is to avoid or decrease the stimulation and damage of relevant organs or tissues caused by various clinical operations, so as to reduce the stress response and discomfort of the body, reduce the risk of postoperative complications, and achieve rapid recovery.
Our results must be evaluated in the context of the study’s limitations. Firstly, we acknowledged that this study was limited by a relatively small population and patient selection bias. Secondly, since the ERAS is optimized to shorten postoperative time and promote patients’ recovery, it needs the cooperation of multiple departments including those of surgery, anesthesia, and nursing. Although our studies have shown that the selection of 3 ERAS measures can benefit the postoperative recovery of pectus excavatum patients, the association between other ERAS measures and these measures, and whether they would have an impact on patients' postoperative recovery, are unclear. Thus, a well-designed randomized control trial is needed to further demonstrate the association of these measures and their influence on postoperative recovery for patients with PE.
Conclusions
The measures of no IDUC, laryngeal mask anesthesia, and indwelling tubule drainage can improve postoperative recovery quality of pectus excavatum patients following Nuss procedure. However, more high-level evidence-based medicine research is needed for the improvement and promotion of the ERAS pathway in pectus excavatum patients.
Acknowledgments
Funding: (I) The Construction of Liaoning Cancer Research Center (Lung Cancer) (2019JH6/10200011). (II) Technological Special Project of Liaoning Province of China (2019020176-JH1/103). (III) Central financial fund for promoting medical service and safeguarding capability (Capability construction of medical and health organizations) –subsidy to the Construction of Provincial Key Specialty. (IV) Research grant to introduced talents of Liaoning Cancer Hospital.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1516
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1516
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1516). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Liaoning Cancer Hospital Ethics Committee (No. 20200302).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rha EY, Kim JH, Yoo G, et al. Changes in thoracic cavity dimensions of pectus excavatum patients following Nuss procedure. J Thorac Dis 2018;10:4255-61. [Crossref] [PubMed]
- Nuss D, Obermeyer RJ, Kelly RE, et al. Pectus excavatum from a pediatric surgeon's perspective. Ann Cardiothorac Surg 2016;5:493-500. [Crossref] [PubMed]
- Kelly RE, Shamberger RC, Mellins RB, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg 2007;205:205-16. [Crossref] [PubMed]
- Molik KA, Engum SA, Rescorla FJ, et al. Pectus excavatum repair: experience with standard and minimal invasive techniques. J Pediatr Surg 2001;36:324-8. [Crossref] [PubMed]
- Redding GJ, Kuo W, Swanson JO, et al. Upper thoracic shape in children with pectus excavatum: impact on lung function. Pediatr Pulmonol 2013;48:817-23. [Crossref] [PubMed]
- Nuss D, Kelly RE, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [Crossref] [PubMed]
- Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013;37:259-84. [Crossref] [PubMed]
- Di Rollo D, Mohammed A, Rawlinson A, Douglas-Moore J, Beatty J. Enhanced recovery protocols in urological surgery: a systematic review. Can J Urol 2015;22:7817-23. [PubMed]
- Schukfeh N, Reismann M, Ludwikowski B, et al. Implementation of fast-track pediatric surgery in a German nonacademic institution without previous fast-track experience. Eur J Pediatr Surg 2014;24:419-25. [PubMed]
- Wharton K, Chun Y, Hunsberger J, et al. Successful Use of an Enhanced Recovery After Surgery (ERAS) Pathway to Improve Outcomes Following the Nuss Procedure for Pectus Excavatum. J Pediatr Surg 2020;55:1065-71. [Crossref] [PubMed]
- Nuss D, Kelly RE. Indications and technique of Nuss procedure for pectus excavatum. Thorac Surg Clin 2010;20:583-97. [Crossref] [PubMed]
- Shi R, Xie L, Chen G, et al. Surgical management of pectus excavatum in China: results of a survey amongst members of the Chinese Association of Thoracic Surgeons. Ann Transl Med 2019;7:202. [Crossref] [PubMed]
- Brochhausen C, Turial S, Müller FKP, et al. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg 2012;14:801-6. [Crossref] [PubMed]
- Croitoru DP, Kelly RE, Goretsky MJ, et al. The minimally invasive Nuss technique for recurrent or failed pectus excavatum repair in 50 patients. J Pediatr Surg 2005;40:181-6; discussion 186-7. [Crossref] [PubMed]
- Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg 2010;252:1072-81. [Crossref] [PubMed]
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [Crossref] [PubMed]
- Adamina M, Kehlet H, Tomlinson GA, et al. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery 2011;149:830-40. [Crossref] [PubMed]
- Thiele RH, Rea KM, Turrentine FE, et al. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg 2015;220:430-43. [Crossref] [PubMed]
- Leeds IL, Boss EF, George JA, et al. Preparing enhanced recovery after surgery for implementation in pediatric populations. J Pediatr Surg 2016;51:2126-9. [Crossref] [PubMed]
- Reismann M, Arar M, Schukfeh N, et al. Feasibility of fast-track elements in pediatric surgery. Eur J Pediatr Surg 2012;22:40-4. [Crossref] [PubMed]
- Hoksch B, Kocher G, Vollmar P, et al. Nuss procedure for pectus excavatum in adults: long-term results in a prospective observational study. Eur J Cardiothorac Surg 2016;50:934-9. [Crossref] [PubMed]
- Roberts ST, Patel K, Smith SR. Impact of avoiding post‐operative urinary catheters on outcomes following colorectal resection in an ERAS programme: no IDUC and ERAS programmes. ANZ J Surg 2018;88:E390-4. [Crossref] [PubMed]
- Mavi J, Moor DL. Anesthesia and analgesia for pectus excavatum surgery. Anesthesiol Clin 2014;32:175-84. [Crossref] [PubMed]
- Liu W, Kong D, Yu F, et al. A simple technique for pectus bar removal using a modified Nuss procedure. J Pediatr Surg 2013;48:1137-41. [Crossref] [PubMed]
- Du X, Mao S, Cui J, et al. Use of laryngeal mask airway for non-endotracheal intubated anesthesia for patients with pectus excavatum undergoing thoracoscopic Nuss procedure. J Thorac Dis 2016;8:2061-7. [Crossref] [PubMed]
- Pawlak K, Gasiorowski L, Gabryel P, et al. Analyzing Effectiveness of Routine Pleural Drainage After Nuss Procedure: A Randomized Study. Ann Thorac Surg 2017;104:1852-7. [Crossref] [PubMed]