Ginsenoside metabolite compound K enhances the efficacy of cisplatin in lung cancer cells
Introduction
Panax Ginseng has been widely used as a preventive and/or therapeutic herbal medicine, especially in oriental countries, for a long time. Dammarane-type tetracyclic triterpenoid saponins, also known as ginsenosides, are well accepted as the major ingredients responsible for the pharmaceutical functions of ginseng (1). Ginsenosides are categorized into two main classes based on the structure, e.g., the protopanaxadiol- and protopanaxatriol-families. The difference between these two families is the presence of the hydroxy group at C-6 position (1). After orally taken, ginsenosides are metabolized in intestine by bacteria to produce several active metabolites that have been shown to possess anti-tumor effects in various types of cancer through modulations of different signaling pathways (2-4).
As a major metabolite of protopanaxadiol type ginsenosides, compound K (CK) is one of the best-studied for its anti-cancer activities. It has been reported that CK could inhibit the proliferation of myeloid leukemia, pulmonary adenocarcinoma, gastric adenocarcinoma and hepatoma cell lines (5-7). In addition, the activity of apoptosis induction and metastasis suppression by CK was also described in several cancer cell lines (8-11). Interestingly, CK was shown to inhibit colon cancer growth, which is attributable to its ability to activate p53, a very important tumor suppressor (12). These findings suggest the great potential of CK for cancer intervention.
Cisplatin was the first platinum compound approved by FDA for cancer treatment (13). It has been well documented that p53 is the pivotal effector of cisplatin-mediated anti-tumor effects (14). Although cisplatin is one of the most effective chemotherapies in a variety of cancers, occurrence of drug resistance and considerable side effects make it imperative to develop less toxic and more effective approaches to overcome these limitations (13,14). Given the facts that CK is well tolerated and has minimal side effects (15), in this study, we tested the combinatory effects of CK and cisplatin at low concentrations using lung cancer cell lines.
Materials and methods
Cell lines and reagents
H460, A549 and H1299 cell lines were purchased from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 µg/mL streptomycin (Invitrogen), at 37 °C in the presence of 5% CO2. CK (99% purity) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP, Beijing, China). Cisplatin was purchased from Sigma (St. Louis, MO, USA). The antibodies used for Western Blotting were from Santa Cruz (anti-p53, DO-1; anti-p21, CP-19) and Millipore (anti-glyceraldehyde-3-phosphate dehydrogenase, GAPDH). Control small interference RNA (siRNA) and siRNA against p53 were purchased from Ambion and transfected into cells at 40 nM using Lipofectamine 2000 (Invitrogen) per manufacture’s protocol.
p53 reporter assay
The cignal p53 pathway report assay kit (LUC) was purchased from Qiagen and the assay was performed according to the manufacture’s instruction. Briefly, the cells were transfected with the mixture of inducible p53-resonsive firefly luciferase reporter construct and constitutively expressing renilla construct (40:1) the day after seeding. Three hours after transfection, the cells were split into 96-well plate and allowed to recover for 24 hr followed by treatment with indicated agents for 24 hr. Dual-luciferase assay was then carried out using Dual-Glo Luciferase Assay System (Promega) per manufacture’s protocol.
MTT assay and combination index (CI) calculation
A total of 5,000 cells/well were seeded in 96-well plates and allowed to attach overnight. The cells were then treated with indicated concentrations of CK and cisplatin individually or in combination for 48 hr. MTT was added to the cells and incubated for 2 hr at 37 °C before the absorbance was measured at 450 nm using a microplate reader. The CI value was calculated by using the Calcusyn software (Biosoft, Cambridge, UK) based on the median-effect principle as previously reported in details (16). A CI value <1, =1 or >1 denotes synergism, additivity or antagonism, respectively.
TUNEL assay
The In situ Cell Death Detection Kit-TMR (Roche) was used to detect cell apoptosis following the manufacture’s instruction. The pictures were taken under a fluorescent microscope and the percentage of positive cells was calculated using Image J software (NIH). Four fields were randomly chosen from each well of 12-well plates for the analysis.
Statistical analysis
The student’s two-tailed t-test was employed to determine the difference between control and treatment groups and the P value less than 0.05 was considered statistically significant. Data are presented as mean ± SD.
Results
Compound K (CK) enhances cisplatin-induced p53 expression and activity in lung cancer cells
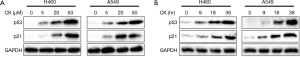
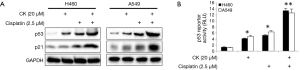
CK was previously shown to induce p53 in colon cancer cells (12).Consistent with this finding, p53 and its target gene p21 levels in large-cell lung cancer cell line H460 and lung carcinoma cell line A549 were induced by CK in a dose- and time-dependent manner (Figure 1A,B). In order to test whether CK could potentially enhance p53 induction by cisplatin, we chose 20 µM CK and 2.5 µM cisplatin, relative low concentrations of these two drugs as indicated by moderate induction of p53, to treat H460 and A549 cells either alone or in combination for 18 hr. Strikingly, as shown in Figure 2A, p53 levels were dramatically increased in the cells treated with both drugs as compared to each treatment alone. In line with this observation, the levels of p21 were also more significantly upregulated by the co-treatment. We further confirmed the enhancement of p53 activity by CK and cisplatin combination treatment by employing p53 Reporter Assay Kit, which monitors the luciferase activity under the control of p53-responsive elements. As shown in Figure 2B, consistent with our Western blot data, 2.5 µM cisplatin and 20 µM CK induced p53 activity by 4-6 folds in these two cell lines, while the co-treatment enhanced p53 induction up to 12-14 folds. These data strongly suggest that CK could enhance p53 expression and activity induced by cisplatin.
Compound K (CK) synergizes cisplatin to inhibit lung cancer cell growth
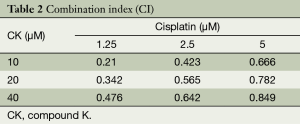
We next attempted to find out the synergistic effect of CK and cisplatin on cell growth by using MTT assay. We tested the cell growth inhibition by treating H460 cells with a serial dilution of CK and cisplatin either alone or in combination for 72 hr. As shown in Table 1, CK or cisplatin single treatment caused dose-dependent cytotoxicity on H460 cells, while the co-treatment led to more profound cell growth inhibition. In order to address whether the enhanced effect is additive or synergistic, the analysis of the CI value was performed. The results in Table 2 showed that the CI values in each combination are all lower than 1, indicating the synergistic effect of these two drugs on cancer cell growth inhibition.

Full table
Full table
Compound K (CK) synergizes cisplatin to induce apoptosis in lung cancer cells
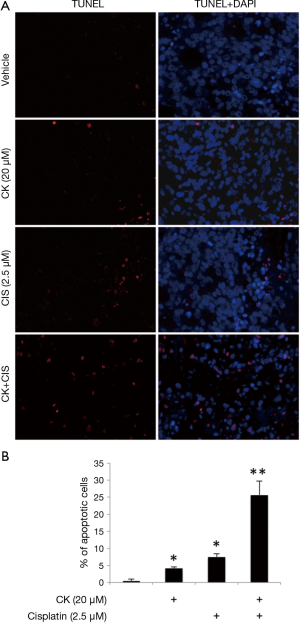
Since cell apoptosis is one of the major mechanisms for p53’s anti-tumor effect, we further tested the induction of apoptosis by either individual or combination treatment of CK and cisplatin using TUNEL assay. Figure 3A showed the representative pictures in each treatment group of H460 cells. The percentage of apoptotic cells were counted and results presented in Figure 3B. In line with the above observations, 20 µM CK and 2.5 µM cisplatin alone caused 4-8% apoptotic cells in these cells, but the co-treatment increased apoptosis to ~25%, suggesting a dramatically enhanced induction of apoptosis.
The synergistic anti-tumor effect of compound K (CK) and cisplatin is p53 dependent
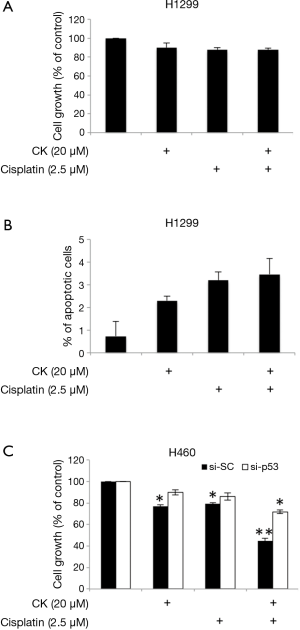
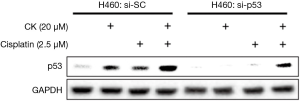
In order to find out whether p53 plays a major role in mediating the synergistic effect of CK and cisplatin, we then tested the cell growth inhibition of these two drugs on H1299, which is a p53-null metastasis lung cancer cell line. As shown in Figure 4A, as expected, treatment with either single agent or combination of CK and cisplatin at the same doses which showed combinatory effect in H460 and A549 cells did cause a slight inhibition on H1299 cell growth. However, the co-treatment failed to produce more profound effect. Consistently, the induction of apoptosis by the co-treatment was also significantly compromised in H1299 cells as compared to H460 cells (Figure 4B). We further evaluated the impact of p53 knockdown on the combinatory effect of CK and cisplatin in H460 cells. As presented in Figure 4C, single and combination treatment showed similar growth inhibition on the control siRNA (si-SC) transfected cells as on the non-transfected cells (Table 1). In contrast, after p53 knockdown (si-p53), the cells were significantly less responsive to the treatment, especially the combination, although the cell growth was also inhibited by CK and cisplatin co-treatment by ~28%, which is very likely attributed to the incomplete knockdown of p53 (Figure S1). Taken together, these data suggest that the synergistic effect of CK and cisplatin depends on the presence of p53.
Discussion
Panax Ginseng is a traditional medicinal herb that has been used in oriental countries for thousands of years. The pharmaceutical effects of ginseng include improving the functions of cerebra, immune system and liver, adjusting blood pressure, anti-fatigue and anti-stress (17). A case-control study showed that the intake of ginseng significantly reduced the risk of various types of cancer (18). Studies from the last two decades revealed the promising anti-tumor effects of ginseng, especially the functionally active ingredients, ginsenosides and their intestinal metabolites (2-4,11). As a major ginsenoside metabolite, CK has demonstrated impressive activities in modulating pathways involved in cancer cell proliferation, apoptosis and metastasis (9,19-21). However, the potential of CK to enhance the efficacy of chemotherapies is less elucidated, especially in lung cancer.
In this study, we showed that when combined with cisplatin, CK significantly increased p53 expression and activity (Figure 2). Consequently, the inhibition of cell growth and the induction of cell apoptosis were enhanced by CK (Table 1 and Figure 3). The CI calculation indicates that the growth inhibition effect is synergistic, rather than additive (Table 2). By using p53-null cell line, our data also suggest that the synergistic effect is p53 dependent (Figure 4). Together, our findings for the first time show the effect of CK to synergistically improve the outcome of cisplatin in a p53-dependent manner, and thus provide strong rationale to develop CK as an adjuvant drug for cisplatin-based lung cancer intervention.
Conventional chemotherapies are often accompanied by development of drug resistance and undesired side effects. Natural compounds with minimum toxicity have drawn significant attentions for their potential use in cancer intervention (22), and possible combination treatments that could lower the dose of chemotherapeutic drugs but maintain or improve the efficacy are of great interest. CK is well tolerated and has very low toxicity and minimal side effects (15), thus making it a promising candidate for this purpose, while our present study well supports the potential. Future investigations will focus on the in vivo synergistic effects and possibly expand the test of CK with other chemotherapy drugs. Detailed mechanisms underlying the synergy are also desired.
Acknowledgements
Funding: This work was supported by the following grants: National Natural Science Foundation of China (81401883); the First Hospital of Jilin University 4th Young Investigator Grant (JDYY42013008).
Disclosure: The authors declare no conflict of interest.
References
- Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci 2004;95:153-7. [PubMed]
- Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev 2004;9:259-74. [PubMed]
- Kim HS, Lee EH, Ko SR, et al. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch Pharm Res 2004;27:429-35. [PubMed]
- Cao B, Liu X, Li J, et al. 20(S)-protopanaxadiol-aglycone downregulation of the full-length and splice variants of androgen receptor. Int J Cancer 2013;132:1277-87. [PubMed]
- Cho SH, Chung KS, Choi JH, et al. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer 2009;9:449. [PubMed]
- Lee SJ, Sung JH, Lee SJ, et al. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Lett 1999;144:39-43. [PubMed]
- Hu C, Song G, Zhang B, et al. Intestinal metabolite compound K of panaxoside inhibits the growth of gastric carcinoma by augmenting apoptosis via Bid-mediated mitochondrial pathway. J Cell Mol Med 2012;16:96-106. [PubMed]
- Zheng ZZ, Ming YL, Chen LH, et al. Compound K-induced apoptosis of human hepatocellular carcinoma MHCC97-H cells in vitro. Oncol Rep 2014;32:325-31. [PubMed]
- Law CK, Kwok HH, Poon PY, et al. Ginsenoside compound K induces apoptosis in nasopharyngeal carcinoma cells via activation of apoptosis-inducing factor. Chin Med 2014;9:11. [PubMed]
- Kang KA, Piao MJ, Kim KC, et al. Compound K, a metabolite of ginseng saponin, inhibits colorectal cancer cell growth and induces apoptosis through inhibition of histone deacetylase activity. Int J Oncol 2013;43:1907-14. [PubMed]
- Wang H, Jiang D, Liu J, et al. Compound K induces apoptosis of bladder cancer T24 cells via reactive oxygen species-mediated p38 MAPK pathway. Cancer Biother Radiopharm 2013;28:607-14. [PubMed]
- Zhang Z, Du GJ, Wang CZ, et al. Compound K, a Ginsenoside Metabolite, Inhibits Colon Cancer Growth via Multiple Pathways Including p53-p21 Interactions. Int J Mol Sci 2013;14:2980-95. [PubMed]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007;7:573-84. [PubMed]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014;740:364-78. [PubMed]
- Gao YL, Liu ZF, Li CM, et al. Subchronic toxicity studies with ginsenoside compound K delivered to dogs via intravenous administration. Food Chem Toxicol 2011;49:1857-62. [PubMed]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984;22:27-55. [PubMed]
- Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin 2008;29:1109-18. [PubMed]
- Yun TK, Choi SY. Preventive effect of ginseng intake against various human cancers: a case-control study on 1987 pairs. Cancer Epidemiol Biomarkers Prev 1995;4:401-8. [PubMed]
- Chen Y, Xu Y, Zhu Y, et al. Anti-cancer effects of ginsenoside compound k on pediatric acute myeloid leukemia cells. Cancer Cell Int 2013;13:24. [PubMed]
- Song G, Guo S, Wang W, et al. Intestinal metabolite compound K of ginseng saponin potently attenuates metastatic growth of hepatocellular carcinoma by augmenting apoptosis via a Bid-mediated mitochondrial pathway. J Agric Food Chem 2010;58:12753-60. [PubMed]
- Choo MK, Sakurai H, Kim DH, et al. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappaB signaling in murine colon cancer cells. Oncol Rep 2008;19:595-600. [PubMed]
- Choi EJ, Kim GH. Antioxidant and anticancer activity of Artemisia princeps var. orientalis extract in HepG2 and Hep3B hepatocellular carcinoma cells. Chin J Cancer Res 2013;25:536-43. [PubMed]


